��Ŀ����
����Ŀ������к͵ζ�����ѧ��ѧ����ʵ�顣
��.��ͼ��ʾ50mL��ʽ�ζ�����Һ���λ�ã����Һ�洦�Ķ�����a����ζ�����ʣ��Һ��������______mL��
A��a B������a C����(50-a) D������(50-a)
��.ijͬѧ���ⶨijŨ������Ʒ�����ʵ���Ũ�ȣ�����������ʵ�������
A ��ȴ�����º���100mL����ƿ�ж������100mLϡ���ᡣ
B ��ȡ20.00mLϡ��������ƿ�в����뼸��ָʾ����
C ����ʽ�ζ��ܺͼ�ʽ�ζ���������ˮϴ�Ӹɾ������ø���ʢ��Һ��ϴ��
D �����ʵ���Ũ��Ϊ1.50 mol��L��1�ı�NaOH��Һװ���ʽ�ζ��ܣ�����Һ����¶���V1��
E �����ζ����յ㣬���¶���ΪV2��
F ����ƿ�µ�һ�Ű�ֽ������ƿ�Ƶ���ʽ�ζ�����С�ĵ���NaOH����Һ���ߵα�ҡ����ƿ��
G ��ȡŨ������Ʒ5 mL�����ձ���������ˮ�ܽ⡣
H �ظ�����ʵ�顣
��ش��������⣺
��1����ʵ����ȷ���������˳��Ϊ____�� A ��_____��_____��D��_____��_____�� H���ñ����ĸ��д����
��2����ȡ5mLŨ�����������________________________����ȡ20.00mLϡ�����������_________��
��3��ѡ�õ�ָʾ����_____________���ζ������У�����Ӧע��____________________���жϵ���ζ��յ��������________________������ʱ������Ӧ____________������ڡ��������ڡ�����ƽ�ڡ�����Һ��Һ�����ʹ���
��4���±���ʵ���õ��й����ݣ�
�ζ���� | ����ϡ��������(mL) | ������NaOH����ҺҺ�����(mL) | |
V1 | V2 | ||
�� | 20��00 | 0��50 | 22��60 |
�� | 20��00 | 6��00 | 27��90 |
��������Ũ������Ʒ��Ũ��Ϊ____________mol��L��1 (����д���������)��
���𰸡�D G C B F E ��Ͳ ��ʽ�ζ��� ��̪����� ��ƿ����Һ��ɫ�ı仯 ��Һ����ɫ��ɺ�ɫ������Һ�ɺ�ɫ��ɳ�ɫ����30s�ڲ���ɫ ��ƽ�� 16.5
��������
��. ���ݵζ��̶ܿ�ֵ���ϵ��¿̶��������Լ�����ԭ����ע��ζ������̶��·��̶ȣ�
��1�������к͵ζ�ԭ����ʵ��Ҫ���������
��2����Ͳ��ȷ��Ϊ0.1mL���ζ��ܾ�ȷ��Ϊ0.01mL��
��3������ǿ��ǿ���γ����ԣ�Ӧѡ�����Ի���Է�Χ�ڱ�ɫ��ָʾ��������Ȼ��̪�����ݵζ������У�Ŀ��Ӧע����ƿ����Һ��ɫ�ı仯���Ա�ȷ�ж��յ�ĵ������ʱӦƽ����Һ��Һ�����ʹ���
��4����������ʵ�������������Ƶ������ȡƽ��ֵ����������кͷ�Ӧn��OH-��=n��H+������Ũ��������ʵ���Ũ�ȡ�
��. �ζ��̶ܿ�ֵ���ϵ��¿̶����������ڵζ������̶��·��̶ȣ�50mL�ζ�����ʵ��ʢ��Һ����������50ml�����Һ�洦�Ķ�����a����ζ�����Һ����������(50a)mL����ѡD��
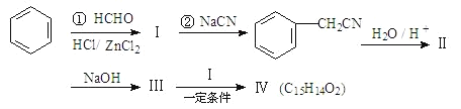
��. (1)����ʽ�ζ���ȷ��ȡŨ������Ʒ10.00mL�����ձ���������ˮ�ܽ⣬��ȴ�����º�,��250mL����ƿ�ж������250mLϡ���ᣬ����Һ����ȡ25.00mLϡ��������ƿ�в����뼸��ָʾ���������ʵ���Ũ��ΪM mol/L�ı�NaOH��Һװ���ʽ�ζ���,����Һ��,���¿�ʼ����ΪV1������ƿ�µ�һ�Ű�ֽ���ζ����յ�,���¶���V2������ȷ�IJ���˳��Ϊ��G��A��C��B��D��F��E��H��
�ʴ�Ϊ��G��C��B��F��E��
(2)��Ͳ��ȷ��Ϊ0.1mL���ζ��ܾ�ȷ��Ϊ0.01 mL��������ȡ5mLŨ�������������Ͳ����ȡ20.00mLϡ����������ǣ���ʽ�ζ��ܣ��ʴ�Ϊ����Ͳ����ʽ�ζ��ܣ�
(3)������������Ʒ�Ӧ���������ƣ���������Һ�����ԣ�Ӧѡ�����Ի���Է�Χ�ڱ�ɫ��ָʾ�������̪����ȣ��ڵζ������У�Ŀ��Ӧע����ƿ����Һ��ɫ�ı仯�������м����̪����Һ��ʾ��ɫ�����ᷴӦ��ȫ��������������Һ����Һ��ʾ��ɫ�Ұ�����ڲ���ʧ������ʱ������Ӧ��ƽ����Һ��Һ�����ʹ����ʴ�Ϊ����̪����ȣ���ƿ����Һ��ɫ�ı仯����Һ����ɫ��ɺ�ɫ������Һ�ɺ�ɫ��ɳ�ɫ����30s�ڲ���ɫ����ƽ�ڣ�
(4)�������������Ƶ����Ϊ��22.600.50=22.10 (mL)��
�������������Ƶ����Ϊ��27.906.00=21.90 (mL)��
����������������Һ�����ƽ��ֵΪ��(22.10+21.90) ��2 = 22.00 (mL)��
��������к͵ζ�ԭ��n(OH)=n(H+)��1.50molL1��22.00mL = 20.00ml��2c(ϡ)���������ϡ�����Ũ��c(ϡ) = 0.825mol/L���ٸ���ϡ����c(ϡ)V(ϡ)=c(Ũ)V(Ũ)��֪����Ũ������Ʒ��Ũ��Ϊc(Ũ)=![]() =16.5mol/L��
=16.5mol/L��

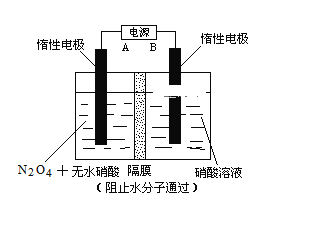
����Ŀ��N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע����֪N2O5����ˮ������Ӧ�����ų��������ȡ�
��.һ���¶��£��������ܱ�������N2O5�������з�Ӧ��2N2O5(g)![]() 4NO2(g)��O2(g)��H��
4NO2(g)��O2(g)��H��
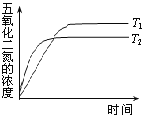
��1���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
t/s | 0 | 500 | 1000 |
c(N2O5)/mol��L��1 | 5.00 | 3.52 | 2.48 |
��500s��NO2����������Ϊ______________��
��2����Ӧ�ﵽƽ�������ͨ��һ����N2O5���ﵽ��ƽ��ʱ��N2O5��ת���ʽ�______�����������С���������䡱����
��3�����¶�T1��T2ʱ��N2O5��Ũ���뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ���ݴ��жϣ�T1______T2���>������<����=������ͬ������H______0��
��.��ͼ��ʾװ�ã��������Ʊ�N2O5���塣A�ǵ�Դ��____________������������������������ص�������ӦʽΪ______________________________________________________��
����Ŀ���о�����CO2�ŷ���һ����Ҫ���⡣CO2��������������ɵ�̼�л����Ҫ�����·�Ӧ��
��Ӧ����CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H1����49.6 kJ/mol
CH3OH(g)��H2O(g) ��H1����49.6 kJ/mol
��Ӧ����CH3OCH3(g)��H2O(g) ![]() 2CH3OH(g) ��H2����23.4 kJ/mol
2CH3OH(g) ��H2����23.4 kJ/mol
��Ӧ����2CO2(g)��6H2(g) ![]() CH3OCH3(g)��3H2O(g) ��H3
CH3OCH3(g)��3H2O(g) ��H3
��1����H3��________kJ/mol��
��2�����º��������£����ܱ�������ͨ������ʵ�����CO2��H2��������ӦI������������˵����ӦI�ﵽƽ��״̬����_______������ţ���
A����Ӧ��ϵ��ѹǿ���ֲ���
B�������ڵĻ��������ܶȱ��ֲ���
C��ˮ�����ж���2NA��H-O����ͬʱ������ж���3NA��H-H��
D��CH3OH��H2O��Ũ��֮�ȱ��ֲ���
��3����ӦII��ij�¶��µ�ƽ�ⳣ��Ϊ0.25�����¶��£����ܱ������м�������ʵ�����CH3OCH3(g)��H2O(g)����Ӧ��ijʱ�̲�ø����Ũ�����£�
���� | CH3OCH3(g) | H2O(g) | CH3OH(g) |
Ũ��/mol��L��1 | 1.8 | 1.8 | 0.4 |
��ʱv��___v������������������������������������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH�������(CH3OH)% ��___%��
��4����ijѹǿ�£���ӦIII�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ƽ��ת������ͼ��ʾ��T1�¶��£���6mol CO2��12mol H2����2 L���ܱ������У�5min��Ӧ�ﵽƽ��״̬����0��5min�ڵ�ƽ����Ӧ����v(CH3OCH3)��____��KA��KB��KC����֮��Ĵ�С��ϵΪ____��
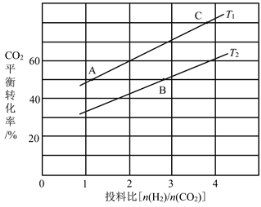
��5����ѹ�½�CO2��H2�������1��3��ϣ��ڲ�ͬ���������·�����ӦI�ͷ�ӦIII������ͬ��ʱ�����CH3OH��ѡ���ԺͲ������¶ȵı仯��ͼ�����У�CH3OH��ѡ���ԣ�![]() ��100%
��100%

�����������ºϳɼ״��Ĺ�ҵ������____��
A��210�� B��230�� C������CZT D������CZ(Zr��1)T