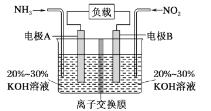
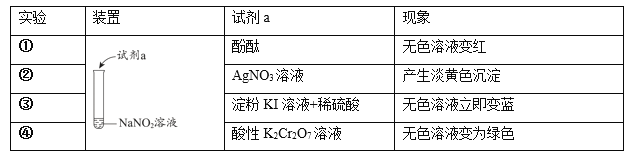
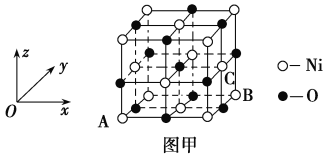
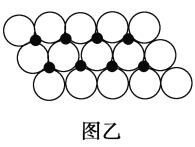
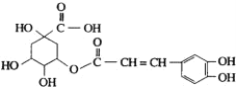
��Ŀ����
����Ŀ��ʵ���ұ�����������Ϊ98%,�ܶ�Ϊ1.84 g��cm-3������,�ݴ�����˵��������� (����)
A. ����������ʵ���Ũ��Ϊ18.4 mol��L-1
B. ������50 mL��������ͭ��Ӧ�ɵõ���״����SO2 0.46 mol
C. ijͬѧ�ø���������ϡ����ʱ,δϴ���ձ��Ͳ�����,������������Ƶ�ϡ����Ũ��ƫ��
D. ��������ˮ���������������Һ�����ʵ���Ũ��С��9.2 mol��L-1
���𰸡�B
��������
A����������Ϊ98%�ܶ�Ϊ1.84gcm-3�����ᣬ���ʵ���Ũ��C=![]() =18.4mol/L����A��ȷ��B��������50mL��������ͭ��Ӧ�����������ȫ��Ӧ�ɵõ���״����SO20.46mol���������ŷ�Ӧ���У�����Ũ�Ƚ��ͱ�Ϊϡ���ᣬϡ������ͭ����Ӧ������ʵ�ʵõ���״����SO2С��0.46mol����B����C��ijͬѧ�ø���������ϡ����ʱ��δϴ���ձ��Ͳ����������²���������ģ����ʵ����ʵ���ƫС���������������Ƶ�ϡ����Ũ��ƫ�ͣ���C��ȷ��D�������Ũ��ԽС�ܶ�ԽС�����������������ˮ���������Һ����Ϊԭ������Һ��2�����ܶ�С��ԭ������Һ���ʻ�Ϻ���Һ���������ԭ������Һ��2����ϡ�ͺ���������䣬������Һ�����ʵ���Ũ��С��9.2mol/L����D��ȷ���ʴ�ΪB��
=18.4mol/L����A��ȷ��B��������50mL��������ͭ��Ӧ�����������ȫ��Ӧ�ɵõ���״����SO20.46mol���������ŷ�Ӧ���У�����Ũ�Ƚ��ͱ�Ϊϡ���ᣬϡ������ͭ����Ӧ������ʵ�ʵõ���״����SO2С��0.46mol����B����C��ijͬѧ�ø���������ϡ����ʱ��δϴ���ձ��Ͳ����������²���������ģ����ʵ����ʵ���ƫС���������������Ƶ�ϡ����Ũ��ƫ�ͣ���C��ȷ��D�������Ũ��ԽС�ܶ�ԽС�����������������ˮ���������Һ����Ϊԭ������Һ��2�����ܶ�С��ԭ������Һ���ʻ�Ϻ���Һ���������ԭ������Һ��2����ϡ�ͺ���������䣬������Һ�����ʵ���Ũ��С��9.2mol/L����D��ȷ���ʴ�ΪB��

����Ŀ�������г����۵�ij��ʳ�þ����ΰ�װ����������˵����
��Ʒ�� | GB5461 |
��Ʒ�ȼ� | һ�� |
�� �� | ʳ�Ρ�����ء������ |
�⺬��(��I�ƣ� | 20~50mg/kg |
��װʱ�� | |
��װ��ҵ |
��1���������⻯�������������·������·�Ӧ����ƽ��ѧ����ʽ������ѧ���������ڿհ״���
____KIO3��___KI��___H2SO4��___K2SO4��___I2��___H2O
��2��������Ӧ���ɵ�I2�������Ȼ�̼���顣�������Ȼ�̼��Һ�м���Na2SO3ϡ��Һ����I2��ԭ���Ի������Ȼ�̼��
��Na2SO3ϡ��Һ��I2��Ӧ�����ӷ���ʽ��_________________________________��
��ijѧ����ƻ������Ȼ�̼�IJ�������Ϊ��
a.��������Ȼ�̼��Һ���ڷ�Һ©���У�
b.��������Na2SO3ϡ��Һ��
c.������²�Һ�塣
�����������©�IJ����������������е�λ����______________________��
��3����֪��I2��2S2O32����2I����S4O62����ijѧ���ⶨʳ�þ����εĵ⺬�����䲽��Ϊ��
a. ȷ��ȡwgʳ�Σ�����������ˮʹ����ȫ�ܽ⣻
b.��ϡ�����ữ������Һ����������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��
c.�Ե���Ϊָʾ������μ������ʵ���Ũ��Ϊ2.0��10��3mol��L��1��Na2S2O3��Һ10.0mL��ǡ�÷�Ӧ��ȫ��
���ж�c�з�Ӧǡ����ȫ���ݵ�������______________________��
��b�з�Ӧ��������I2�����ʵ�����___________mol��
����������ʵ��Ͱ�װ��˵�������⾫���εĵ⺬���ǣ��Ժ�w�Ĵ���ʽ��ʾ��
_______________________mg/kg��
����Ŀ������к͵ζ�����ѧ��ѧ����ʵ�顣
��.��ͼ��ʾ50mL��ʽ�ζ�����Һ���λ�ã����Һ�洦�Ķ�����a����ζ�����ʣ��Һ��������______mL��
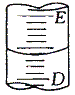
A��a B������a C����(50-a) D������(50-a)
��.ijͬѧ���ⶨijŨ������Ʒ�����ʵ���Ũ�ȣ�����������ʵ�������
A ��ȴ�����º���100mL����ƿ�ж������100mLϡ���ᡣ
B ��ȡ20.00mLϡ��������ƿ�в����뼸��ָʾ����
C ����ʽ�ζ��ܺͼ�ʽ�ζ���������ˮϴ�Ӹɾ������ø���ʢ��Һ��ϴ��
D �����ʵ���Ũ��Ϊ1.50 mol��L��1�ı�NaOH��Һװ���ʽ�ζ��ܣ�����Һ����¶���V1��
E �����ζ����յ㣬���¶���ΪV2��
F ����ƿ�µ�һ�Ű�ֽ������ƿ�Ƶ���ʽ�ζ�����С�ĵ���NaOH����Һ���ߵα�ҡ����ƿ��
G ��ȡŨ������Ʒ5 mL�����ձ���������ˮ�ܽ⡣
H �ظ�����ʵ�顣
��ش��������⣺
��1����ʵ����ȷ���������˳��Ϊ____�� A ��_____��_____��D��_____��_____�� H���ñ����ĸ��д����
��2����ȡ5mLŨ�����������________________________����ȡ20.00mLϡ�����������_________��
��3��ѡ�õ�ָʾ����_____________���ζ������У�����Ӧע��____________________���жϵ���ζ��յ��������________________������ʱ������Ӧ____________������ڡ��������ڡ�����ƽ�ڡ�����Һ��Һ�����ʹ���
��4���±���ʵ���õ��й����ݣ�
�ζ���� | ����ϡ��������(mL) | ������NaOH����ҺҺ�����(mL) | |
V1 | V2 | ||
�� | 20��00 | 0��50 | 22��60 |
�� | 20��00 | 6��00 | 27��90 |
��������Ũ������Ʒ��Ũ��Ϊ____________mol��L��1 (����д���������)��