��Ŀ����
����Ŀ��ͨ����ú���ۺ����ÿɵõ��ྻ��ȼ�ϺͶ��ֻ���ԭ�ϣ�Ҳ�ɼ��ٻ�������Ⱦ��ú�ļ��Һ���ɵõ��״���
��1����֪��CH3OH��H2��ȼ���ȣ���H���ֱ�Ϊ��726.5kJ/mol����285.8kJ/mol��������CO2��H2��Ӧ����CH3OH��H2O���Ȼ�ѧ����ʽ��____________��
��2��һ�������£�CO��H2�ϳ�CH3OH��CO(g)+2H2(g)![]() CH3OH(g)��
CH3OH(g)��
�������һ�����ܱ������а����ʵ���֮��1:2����CO��H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ��
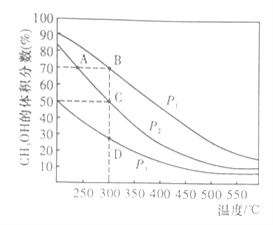
A��B��C����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��___________��ѹǿ��P1____P2�����������������=����
�淴Ӧ���ʣ�v��(A)______v��(B)�����������������=����
��C�㣬CO��ת����Ϊ__________��
��C�㣬���ٰ����ʵ���֮��1:2����һ������CO��H2���������������䣬�ﵽ�µ�ƽ��ʱ��CH3OH���������__________�����������С�����䡱����
�����ں��º��������£��ܱ�ʾ�ÿ��淴Ӧ�ﵽƽ��״̬����__________��
A. ���������ܶȱ��ֲ���
B. ��������ƽ����Է����������ֲ���
C. �����ڵ�ѹǿ���ֲ���
D. ��λʱ����ÿ����1molCO��ͬʱ������2molH2
E. CO��H2��CH3OH��Ũ�ȱ��ֲ���
F. CO��H2��CH3OH��Ũ��֮��Ϊ1:2:1
��3�������ᡢ̼���Ϊ��Ԫ���ᣬ�䳣���µĵ��볣�����±���
H2CO3 | H2S | |
Ka1 | 4.4��10��7 | 1.3��10��7 |
Ka2 | 4.7��10��11 | 7.1��10��15 |
ú�����������в������к�����H2S����������Na2CO3��Һ���գ��÷�Ӧ�����ӷ���ʽΪ________�������£�0.1mol��L��1NaHCO3��Һ��0.1mol��L��1NaHS��Һ��pH��ȣ�pH��С��Ϊ______��Һ���ѧʽ����
���𰸡�CO2(g)+3H2(g)=CH3OH(l)+H2O(l) ��H=��130.9kJ��mol��1KA��KB=KC����75%����BCDECO32��+H2S=HCO3��+HS��NaHCO3
���������������������Ϊ��ѧ��Ӧԭ���ۺ��⣬�漰ȼ���ȵĸ����˹���ɺ��Ȼ�ѧ����ʽ����д����ѧƽ��״̬���жϡ�ͼ�������ƽ����㣬������ʵĵ���������ˮ���֪ʶ�������ػ���֪ʶ���з�����
��𣺣�1��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų�����������֪��CH3OH��H2��ȼ���ȣ���H���ֱ�Ϊ��726.5kJ/mol����285.8kJ/mol�����У���CH3OH��l��+3/2O2��g��=CO2��g��+2 H2O��l����H=-726.5kJ/mol,��H2(g)+1/2O2(g)==H2O(l) ��H=-285.8kJ/mol,���ݸ�˹���ɣ�����3-���ó�����CO2��H2��Ӧ����CH3OH��H2O���Ȼ�ѧ����ʽ��CO2(g)+3H2(g)=CH3OH(l)+H2O(l) ��H=��130.9kJ��mol��1 ��
��2����Ӱ��ƽ�ⳣ�����������Ϊ�¶ȡ�����ͼ��֪B��C�����¶���ͬ����KB=KC����������������ʱ�������¶ȣ��״������������С��ƽ�������ƶ����������¶ȣ�ƽ�������ȷ�Ӧ�����ƶ�����Ӧ��CO(g)+2H2(g)![]() CH3OH(g)Ϊ���ȷ�Ӧ��KA��KB����A��B��C����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��KA��KB=KC ����Ӧ��CO(g)+2H2(g)
CH3OH(g)Ϊ���ȷ�Ӧ��KA��KB����A��B��C����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��KA��KB=KC ����Ӧ��CO(g)+2H2(g)![]() CH3OH(g)����Ϊ�������ʵ�����С�ķ�Ӧ����������������ʱ������ѹǿ��ƽ�������ƶ����״����������������ͼ��֪ѹǿ��P1��P2���¶�Խ�ߣ���Ӧ����Խ�죻ѹǿԽ��Ӧ����Խ�죬�¶ȶԷ�Ӧ���ʵ�Ӱ���ѹǿ���������淴Ӧ���ʣ�v��(A)��v��(B)������ͼ��֪C��״����������Ϊ50%��������������ʼ����CO��H2�����ʵ����ֱ�Ϊ1mol��2mol��ת����CO�����ʵ���Ϊx����������ʽ������
CH3OH(g)����Ϊ�������ʵ�����С�ķ�Ӧ����������������ʱ������ѹǿ��ƽ�������ƶ����״����������������ͼ��֪ѹǿ��P1��P2���¶�Խ�ߣ���Ӧ����Խ�죻ѹǿԽ��Ӧ����Խ�죬�¶ȶԷ�Ӧ���ʵ�Ӱ���ѹǿ���������淴Ӧ���ʣ�v��(A)��v��(B)������ͼ��֪C��״����������Ϊ50%��������������ʼ����CO��H2�����ʵ����ֱ�Ϊ1mol��2mol��ת����CO�����ʵ���Ϊx����������ʽ������
CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼ��mol��1 2 0
ת����mol��x 2x x
ƽ�⣨mol��1-x 2-2x x
����x/(3-2x)��100%=50%�����x=0.75mol����CO��ת����Ϊ0.75mol/1mol��100%=75%����C�㣬���ٰ����ʵ���֮��1:2����һ������CO��H2���൱������ѹǿ��ƽ�������ƶ����ﵽ�µ�ƽ��ʱ��CH3OH���������������A. ���º��������£����������������淴Ӧ�Ľ��ж��仯�����������ܶȲ��淴Ӧ�Ľ��ж��仯�����������ܶȱ��ֲ��䣬����˵����Ӧ�ﵽƽ��״̬������B. ���ŷ�Ӧ�Ľ��У����������������ֲ��䣬���ʵ�����С������M=m/V�жϻ�������ƽ����Է����������ŷ�Ӧ�Ľ��в��ϼ�С����ƽ����Է�����������ʱ��Ӧ�ﵽƽ��״̬����ȷ��C. ���ŷ�Ӧ�Ľ��У������������ʵ�����С�������ڵ�ѹǿ��С����ѹǿ���ֲ���ʱ��Ӧ�ﵽƽ��״̬����ȷ��D. ��λʱ����ÿ����1molCO��ͬʱ������2molH2�������淴Ӧ������ȣ���Ӧ�ﵽƽ��״̬����ȷ��E. CO��H2��CH3OH��Ũ�ȱ��ֲ��䣬��Ӧ�ﵽƽ��״̬����ȷ��F. CO��H2��CH3OH��Ũ��֮��Ϊ1:2:1��Ӧ��һ��Ϊƽ��״̬������ѡBCDE��
��3���������������ᡢ̼��ĵ��볣��֪���ԣ�H2CO3��H2S��HCO3-��HS-��ú�����������в������к��������ǿ��������ԭ��֪H2S��������Na2CO3��Һ��Ӧ����̼�����ƺ����⻯�ƣ��÷�Ӧ�����ӷ���ʽΪCO32��+H2S=HCO3��+HS��������ε������Ӧ����Խ�������ε�ˮ��̶�Խ����ͬŨ��ʱ����Һ�ļ���Խǿ��pHԽ�ʳ����£�0.1mol��L��1NaHCO3��Һ��0.1mol��L��1NaHS��Һ��pH��ȣ�pH��С��ΪNaHCO3��Һ��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�����Ŀ���±���a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ���������:
ѡ�� | ���� | a | b | c |
|
A | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� | |
B | CO2 | ���� | ̼��� | ����NaHCO3��Һ | |
C | NO | ϡ���� | ͭм | H2O | |
D | Cl2 | Ũ���� | �������� | ����NaCl��Һ |
A. A B. B C. C D. D