��Ŀ����
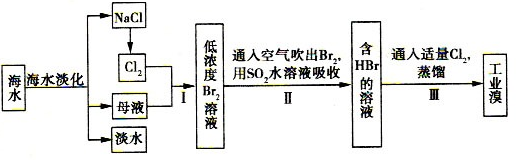
ij����С����Ƴ����ù�ҵ���ᣨ10%H2SO4�����ѽ�ij����������ͭп����Ҫ�ɷݣ�CuO ZnO���ķ�����ʵ�ַ����ۺ����ã���������ͼ��ʾ��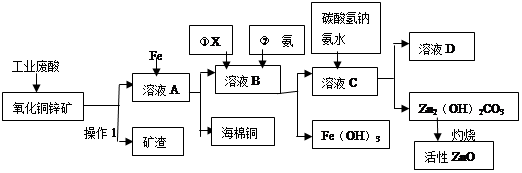
��ش��������⣺
��1������1�������ǣ�
��2������ҺA�м���Fe�۷�������Ҫ�ķ�Ӧ���ӷ���ʽΪ�� �� ��
��3������ҺB�м�������X��Ŀ���� ������X�������������е� ��(����ĸ)
| A��KMnO4 | B��O2 | C��H2O2 | D��NaOH |
��5����ҺD����Ҫ���ʵĻ�ѧʽ�� ��
��6������ͭп���к���������CuS��ZnS����H2SO4��������ZnS�����ܽ��CuS���ܣ�����ͬ�¶��£�Ksp(CuS) Ksp(ZnS)��ѡ�������������������
��1�����ˣ�2�֣�
��2��Fe + 2H+��Fe2+ + H2����2�� Fe + Cu2+��Fe2+ + Cu��2�֣�
��3����Fe2+������Fe3+���Ա��ȥ ��2�֣�B C ��2�֣�
��4��3.2 ��PH�� 6.2��2�֣�
��5��(NH4)2SO4 ��2�֣� ��6���� ��2�֣�
���������������1������1Ϊ��Һ�����������������Ϊ���ˡ�
��2������ͭп�����������������ܽ�����Cu2+��Zn2+��ͬʱΪ��ʹ�������ܽ⣬�������Ҫ������������������ҺΪ������Һ���ټ���Fe�ۣ�������ӦFe + 2H+��Fe2+ + H2���� Fe + Cu2+��Fe2+ + Cu��
��3����Ϊ�õ���B��ҺҪ��������Fe��OH��3 ��������Ѷ���������Ϊ�������Ա���õij�ȥ�����Գ��˼Ӽ���ˮ��Һ�⣬��Ӧ�ü��������������Ҳ��������ʣ����Կ���ѡ��O2��H2O2����ѡBC��
��4�����백ˮ��Ҫ��ʹFe3+��ȫ����������ʹZn2+����������Ӧ�ÿ���PH��Χ��3.2 ��PH�� 6.2֮��
��5�����ڹ��������μ��백ˮ����C��Һ�е�Zn2+��̼������������˳�����ʽ̼��п������ʣ��D��Һ�к��еĴ���������ΪNH4+������������ΪSO42-��������Ҫ����Ϊ(NH4)2SO4 ��
��6����H2SO4��������ZnS�����ܽ��CuS���ܣ�˵��CuS�����ܣ�������ͬ�¶��£�Ksp(CuS)<Ksp(ZnS)��
���㣺���⿼����ǻ��������⡣

���ᣨH3BO3)����Ӧ���ڲ���������ҵ������þ��2Mg0.B203.H20��Si02������Fe304��CaCO3, Al2O3)Ϊԭ����������Ĺ����������£�
��֪��H3BO3��200C��400C��600C��1000Cʱ���ܽ������Ϊ5.0 g��8.7 g��14.8 g��40. 2 g��Fe3 +��Al3+��Fe2 +��Mg2+������������ʽ��ȫ����ʱ����Һ��pH�ֱ�Ϊ3. 2�� 5.2��9.7�� 12.4��
��1�����ڿ���к�CaC03��Ϊ��ֹ����ȡ��ʱ���ײ���������ĭʹ���ϴӷ�Ӧ���������Ӧ��ȡ�Ĵ�ʩ�ǣ� ��
��2��������Һ�������ԣ���H3B03��Mg2+��SO42-��������Fe2+��Fe3+��Ca2+��Al3+�����ʡ������ӡ�ʱ�����Һ�����μ�������H202��Mg0,��ȥ������������_______��H2O2��������____________________________________________ (�����ӷ���ʽ��ʾ����
��3������ȡ�����á��ȹ��ˡ���Ŀ����_____________________________________��
��4����ĸҺ�������ڻ�������þ����֪����þ���ܽ�����¶ȱ仯����������ͼ������ Һ�ķе���ѹǿ��������ߡ�Ϊ�˴ӡ�ĸҺ���г�ֻ���MgS04��H20,Ӧ��ȡ�� ��ʩ�ǽ���ĸҺ������Ũ����____________
��5�����ᣨH3BO3����Һ�д������·�Ӧ��H3BO3(aq)+H2O(l)  [B(OH)4]����aq��+H+(aq) K��5.7��10��10��298K��
[B(OH)4]����aq��+H+(aq) K��5.7��10��10��298K��
����25��ʱ0.7mol��L��1������Һ��H����Ũ�ȡ���д��������̣�
��6����֪298Kʱ��
| ��ѧʽ | ̼�� | ���� |
| ���볣�� | K1=4.4��10��7 K2=4.7��10��11 | K��1.75��10��5 |
����˵����ȷ���� ��
A��̼������Һ�����������ܹ۲쵽�����ݲ���
B��̼������Һ����������ܹ۲쵽�����ݲ���

C����Ũ�ȵ�̼���������Һ�Ƚϣ�pH��ǰ�ߣ�����
D����Ũ�ȵ�̼���ƺʹ�������Һ�Ƚϣ�pH��ǰ�ߣ�����
���������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��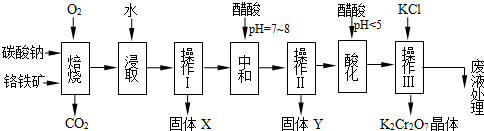
��֪����4FeO��Cr2O3+ 8Na2CO3+ 7O2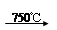 8Na2CrO4 + 2 Fe2O3 + 8CO2����
8Na2CrO4 + 2 Fe2O3 + 8CO2����
��Na2CO3 + Al2O3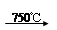 2NaAlO2 + CO2������ Cr2O72��+ H2O
2NaAlO2 + CO2������ Cr2O72��+ H2O 2CrO42�� + 2H+
2CrO42�� + 2H+
��1������X����Ҫ����_________����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ��__________����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH��5����Ŀ����_________________________________��
| ���� | �ܽ��/(g/100gˮ) | ||
| 0��C | 40��C | 80��C | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� ��
���ˡ�_______�����
��4���ұ���������ʵ��ܽ�����ݣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl��K2Cr2O7��+2NaCl���÷�Ӧ����Һ���ܷ�����������_______________��
��5��������Һ�й���������ʹCr2O72-������ɫ�Ĺ���������CrO5���ӽṹΪ
 ), �÷�Ӧ����������Cr2O72-�Ĵ��ڡ�д����Ӧ�����ӷ���ʽ�� ��
), �÷�Ӧ����������Cr2O72-�Ĵ��ڡ�д����Ӧ�����ӷ���ʽ�� ���÷�Ӧ (����ڡ������ڡ�)������ԭ��Ӧ��
��6����ȡ�ظ��������2.500g���250mL��Һ,ȡ��25mL�����ƿ��,����10mL2mol/ L H2SO4�������⻯�أ����Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���I2+2S2O32����2I-+S4O62������
���жϴﵽ�ζ��յ�������� ��
����ʵ���й���ȥNa2S2O3����Һ40.00mL�������ò�Ʒ���ظ���صĴ���Ϊ ��������3λ��Ч����, K2Cr2O7��Ħ������Ϊ294g/mol����
���и������ʵķ�����ᴿ��Ӧѡ��������������һ�֣�����ѡ����ĸ��
| A����Һ | B������ | C����ȡ | D������E�������ᾧ����F�����·ֽ� |
�ڳ�ȥ����ʯ��ˮ��������CaCO3������ �� ������
�۳�ȥCaO������������CaCO3���壺���� �� ������
�ܴӵ�ˮ����ȡ�⣺���� �� ������
�ݷ���CCl4���е�Ϊ76.75�棩�ͼױ����е�Ϊ110.6�棩��Һ��������� �� ������
��16�֣�ijͬѧ���������˽�����и��������Ե��������Σ������κ�̼���Ρ���ͬѧ����ʵ����֤��һ��ʵ��������в����εĺ������������ϵ�֪���ᣨH2C2O4����һ�ֶ�Ԫ�л����ᣬ���н�ǿ�Ļ�ԭ�ԣ�����ƣ�CaC2O4��������ˮ������ϡ���ᡣ��ش��������⣺
��1����ѧ����������ĥ�ɷ�ĩ����ˮ���ݡ����˵õ�������ҺA����ĥ����ʹ�õ�ʵ������������Ϊ ��
��2�����ʵ�鷽����֤�����к��в����κ�̼���Σ��������Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��������ҺA�������ԣ��μ�����CaCl2��Һ�� | �ٳ��ְ�ɫ������˵�������� �� |
| ����2��ȡ����1�ij������Թ��У�����������ϡ���ᣬ������ȫ�ܽ⣬������������ɫ��ζ������ͨ�����ʯ��ˮ�С� | �� �� |
| ����3������2�õ�����Һ�еμӼ��θ��������Һ�� | �� �� |
�ٲ�����ȡm g������Ʒ�������в�����ȫ��ת��ΪCaC2O4����������������ձ��У��ù�����ϡ������ȫ�ܽ����Һת�� �в���ˮ���Ƴ�250mL��Һ��ÿ����ȡ25��00mL����Һ����ƿ�У���0��0100mol��L��1 KMnO4����Һ�ζ���ƽ�����ı���ҺV mL��KMnO4�ζ�ʵ��ʱ���������ӷ���ʽΪ ��
�ڼ��㣺�����в����Σ���C2O42�����㣩����������Ϊ������ֻ��ʽ������ ��
��������: ���в�����ʹ�ⶨ���ƫ�ߵ��� ��
A��������Һʱδϴ���ձ��Ͳ������ͼ�ˮ����
B����ƿδ����ͼ������Һ���еζ�
C��δ�ñ�Һ��ϴ�ͼ����Һ��ʼ�ζ�
D���ζ�ǰ���촦�����ݵζ���������ʧ
E���ζ����Ӷ���

 ZnFe2(C2O4)3��6H2O������������(a)
ZnFe2(C2O4)3��6H2O������������(a) ZnFe2O4+2CO2��+4CO��+6H2O ����������(b)
ZnFe2O4+2CO2��+4CO��+6H2O ����������(b)