��Ŀ����
����Ŀ��ijѧ����0.10 mol��L��1��NaOH��Һ�ζ�ijŨ�ȵ����ᡣ��¼�������£�
ʵ�� ��� | ����Һ ���/mL | ������NaOH��Һ�����/mL | |
�ζ�ǰ����/mL | �ζ������/mL | ||
1 | 20.00 | 0.50 | 20.54 |
2 | 20.00 | 6.00 | 26.00 |
3 | 20.00 | 1.40 | 21.36 |

��1���ζ�ʱѡ�÷�̪��Һ��ָʾ��������жϵζ��ﵽ�յ�____________��
��2���ζ������У��۾�Ӧע��_____________________��
��3����������ʵ���Ũ��Ϊ___________��
��4����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ���Բⶨ�����Ӱ����__________(����ƫ������ƫ����������Ӱ����)��
��5��ijͬѧ����֪ȷŨ�ȵĸ��������Һ�ζ���Һ��Fe2����Ũ�ȣ����������ҺӦʢ����________(����������������)�У��÷�Ӧ�����ӷ���ʽΪ_______________
���𰸡����������һ��NaOH��Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ�� ��ƿ����Һ��ɫ�ı仯�� 0.10 mol��L��1�� ƫ�� �� 5Fe2����MnO4-��8H��===5Fe3����Mn2����4H2O
��������
��1������Ϊ����Һ����������Ϊ��Һ��ָʾ��Ϊ��̪���ζ��յ�Ϊ��ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ������������������Ƶķ�Ӧ�������м��㣻
��2�����ݸ��������Һ����ǿ�����ԣ�Ӧ����ʽ�ζ���ʢ�ŷ�����
��3������ʵ�����ݵ���Ч�Խ��д�����
��4����ϲ�������������Ũ�ȵ�Ӱ�������
(1)��ƿ��Ϊ����ͷ�̪��Һ���ζ��յ�ı仯Ϊ�����������һ��NaOH��Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
(2)�ڵζ��������۾��۲���ƿ����Һ��ɫ�ı仯��
(3)���εζ���Ҫ������������Һ������ֱ�Ϊ��20.54-0.50=20.04mL��,2.00-6.00=20.00 mL��21.36-1.4=19.96 mL�����ε�ƽ��ֵΪ20.00 mL�������Ũ��Ϊ![]() = 0.10 mol��L��1��
= 0.10 mol��L��1��
(4) ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ��������������Һ����������ⶨ�������Ũ��ƫ�ߣ�
(5) ���������Һ����ǿ�����ԣ���������ʽ�ζ���ʢ�ţ�ѡ��ף�������غ��������ӷ�Ӧ���������Ӻ������Ӻ�ˮ�����ӷ���ʽΪ��5Fe2����MnO4-��8H��===5Fe3����Mn2����4H2O��

����Ŀ���¶�Ϊ![]() ʱ���������ݻ���Ϊ
ʱ���������ݻ���Ϊ![]() �ĺ����ܱ������н�������Ӧ��
�ĺ����ܱ������н�������Ӧ��![]() ����Ӧ����
����Ӧ����![]() ��ʵ���ã�
��ʵ���ã�![]() ��
��![]() ��
��![]() ��
��![]() Ϊ���ʳ��������¶�Ӱ�졣ƽ�ⳣ��
Ϊ���ʳ��������¶�Ӱ�졣ƽ�ⳣ��![]() ����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ
����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ![]() ��ѹ
��ѹ![]() ���ʵ�������������˵������ȷ����
���ʵ�������������˵������ȷ����![]()
![]()
���� ��� | ���ʵ���ʼŨ�� | ���ʵ�ƽ��Ũ�� | ||
|
|
|
| |
�� |
| 0 | 0 |
|
�� |
|
|
| |
�� | 0 |
|
| |
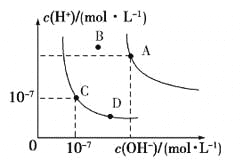
A.��ƽ��ʱ����������![]() ���������е�С
���������е�С
B.������Ӧ��ƽ��ǰ��![]()
C.��ʼʱ����������ѹǿΪ![]() ����
����![]() ʱ�÷�Ӧ��ƽ�ⳣ��
ʱ�÷�Ӧ��ƽ�ⳣ��![]() kPa
kPa
D.���¶ȸı�Ϊ![]() ʱ����
ʱ����![]() ��
��![]()
����Ŀ����֪A(g)+B(g)![]() C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
|�¶�/ �� | 700 | 900 | 830 | 1000 | 1200 |
ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
�ش��������⣺
(1)�÷�Ӧ��ƽ�ⳣ������ʽK=____________����H____0������<���� >���� =��)��
(2)830��ʱ����һ��5 L���ܱ������г���0.20mol��A��0.80mol��B���練Ӧ��ʼ6s��A��ƽ����Ӧ����v(A)=0��003 mol��L-1��s-1����6sʱc(A)=_____mol��L-1�� C�����ʵ���Ϊ______mol������Ӧ��һ��ʱ��ﵽƽ��ʱA��ת����Ϊ_____�������ʱ����ܱ��������ٳ���1 mol�����ƽ��ʱA��ת����Ϊ_________��
(3)�жϸ÷�Ӧ�Ƿ�ﵽƽ�������Ϊ______(����ȷѡ��ǰ����ĸ)��
a��ѹǿ����ʱ��ı� b��������ܶȲ���ʱ��ı�
c��c(A)����ʱ��ı� d����λʱ��������C��D�����ʵ������
(4)1200��ʱ��ӦC(g)+D(g)![]() A(g)+B(g)��ƽ�ⳣ����ֵΪ_____________��
A(g)+B(g)��ƽ�ⳣ����ֵΪ_____________��