��Ŀ����
����Ŀ����ΪʳƷ���Ӽ�ʱ����������(NaNO2)��������������ʶȣ����������������Ʒ�Ľṹ��Ӫ����ֵ�����ǹ�������ᵼ���ж���ijʵ��С�����ʵ���Ʊ��������Ʋ����к����ⶨ��
I��NaNO2�Ʊ�
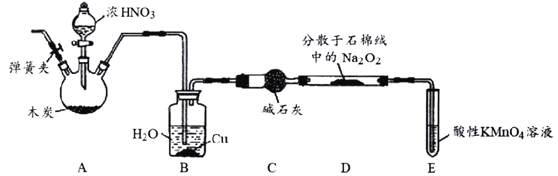
ʵ������ľ̿��Ũ���ᡢNa2O2Ϊ��Ҫԭ�ϰ�����ͼ��ʾװ���Ƹ���������(����װ�ü����ּг�װ������ȥ)����Ӧԭ��Ϊ��2NO+Na2O2=2NaNO2
�ش��������⣺
��1�����Ӻ�װ��֮����һ��ʵ�������______��
��2��Bװ����ͭ��������______��
��3��Eװ������β��������E�з�Ӧ�����ӷ���ʽΪ______��
��4��ʵ������Σ�Ϩ��ƾ���֮�����ͨ��N2ֱ��װ����ȴ����ʱͨ��N2��Ŀ����_____��
�����IJⶨ
�������Ͽ�֪������KMnO4��Һ�ɽ�NO2������ΪNO3����MnO4��ԭ��Mn2+��
��5����Һ���ƣ���ȡװ��D�з�Ӧ��Ĺ���4.000g��������в���ȴ������ˮ���ձ����ܽ⣬��ȫ�ܽ��ȫ��ת����250mL��_____�У�������ˮ��_____��
�ζ���ȡ25.00mL��Һ����ƿ�У���0.1000mol/L����KMnO4��Һ�����ʶ���ʵ�������������±���ʾ��
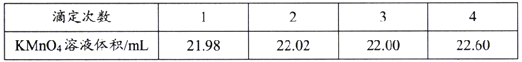
��6����4��ʵ�����ݳ����쳣����������쳣��ԭ�������______(˫��ѡ��)��
A����ƿϴ����δ���� B���ζ��յ����Ӷ���
C���ζ��յ㸩�Ӷ��� D����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
��7�����ݱ������ݣ��������ù������������Ƶ���������______%(����2λС��)��
���𰸡���飨װ�ã������� Cu���Ժ�NO2��H2O��Ӧ���ɵ�HNO3��Ӧ�����NO�IJ��� 5NO+3MnO4-+4H+=5NO3-+3Mn2++2H2O ��װ���е�NOȫ������Eװ�����գ�����ֹ���� ����ƿ �̶��� B��D 94.88
��������
��1��ʵ����������μ�ʱ����װ������������װ�������ԣ�
��2��ͭ���������������ˮ��Ӧ�Ʊ�NO��
��3��β���е�NO���л�ԭ�ԣ��ɱ����Ը�������������գ�
��4�������ɽ�װ���е�NOȫ���Ž�β������װ�ã�����ֹ�������ݴ˷�����
��5��������һ�����ʵ���Ũ�ȵ���Һ�Ļ�������ش�
��6�����ݲ��������Ա�ҺŨ�ȴ�����Ӱ������
��7���������ݿɵ�KMnO4��Һ��ƽ����������ϵ����غ㶨���ҳ���ϵʽ2MnO4-��5NO2-���ݴ˷�������
��1�����ڸ�ʵ����������μӣ���������װ������������Ҫ���װ�õ������ԣ���װҩƷ��
�ʴ�Ϊ����飨װ�ã������ԣ�
��2��װ��B����Aװ�����ɵĶ���������ˮ��Ӧ���������һ��������3NO2+H2O��2HNO3+NO�������ͭ��Ӧ��������ͭ��һ��������ˮ�����Bװ����ͭ��������ͭ���ԺͶ���������ˮ��Ӧ���ɵ����ᷴӦ�����NO���ʣ�
�ʴ�Ϊ�� Cu���Ժ�NO2��H2O��Ӧ���ɵ�HNO3��Ӧ�����NO�IJ��ʣ�
��3�����Ը��������Ϊβ��������Һ����NO����������ԭ��Ӧ�������ӷ���ʽΪ��5NO+3MnO4-+4H+=5NO3-+3Mn2++2H2O��
�ʴ�Ϊ��5NO+3MnO4-+4H+=5NO3-+3Mn2++2H2O��
��4����ΪNO�ж������������Ҫ���õ�����ʣ���NOȫ���������Ը��������Һ�У�����ֹ������
�ʴ�Ϊ����װ���е�NOȫ������Eװ�����գ�����ֹ������
��5��������Һʱ������������ܽ⡢��ȴ����ת�Ƶ�250 mL������ƿ�У��ټ�����ˮ���ٶ������̶��ߣ����ҡ������ǩ��
�ʴ�Ϊ������ƿ���̶��ߣ�
��6�������е�4���Һ�����ƫ����
A. ��ƿϴ����δ����Բⶨ�����Ӱ�죬A�����
B. �ζ��������Ӷ���������ĩ���������������������������ĵ����ƫ��B����ȷ��
C. �ζ��������Ӷ���������ĩ�����ݼ�С�������ֵ��С������������ĵ����ƫС��C�����
D. ��ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ���������ı�Һ�����ƫ�����¸������Ũ��ƫ�ͣ������õ����ƫ��D����ȷ��
�ʴ�Ϊ��B��D��
��7���������ݿɵ�KMnO4��Һ��ƽ�����Ϊ![]() �����ݵ�ʧ�����غ��֪��ϵʽΪ2MnO4-��5NO2-������m��NaNO2��=0.022L��0.1000mol/L��
�����ݵ�ʧ�����غ��֪��ϵʽΪ2MnO4-��5NO2-������m��NaNO2��=0.022L��0.1000mol/L��![]() ��5/2��69 = 3.795 g��������������Ϊ
��5/2��69 = 3.795 g��������������Ϊ![]() =94.875%
=94.875%![]() 94.88%��
94.88%��
�ʴ�Ϊ��94.88��

����Ŀ��Bodensteins�о������з�Ӧ��2HI(g)![]() H2(g)+I2(g) H=+11 kJ��mol-1����716Kʱ�����������е⻯������ʵ�������x(HI)�뷴Ӧʱ��t�Ĺ�ϵ���±���
H2(g)+I2(g) H=+11 kJ��mol-1����716Kʱ�����������е⻯������ʵ�������x(HI)�뷴Ӧʱ��t�Ĺ�ϵ���±���
t/min | 0 | 20 | 40 | 60 | 80 | 120 |
x(HI) | 1 | 0.91 | 0.85 | 0.815 | 0.795 | 0.784 |
x(HI�� | 0 | 0.60 | 0.73 | 0.773 | 0.780 | 0.784 |
������ʵ�����ݼ���õ�v����x(HI)��v����x(H2)�Ĺ�ϵ������ͼ��ʾ�����ı��������ٴδﵽƽ��ʱ�������й���������ȷ����
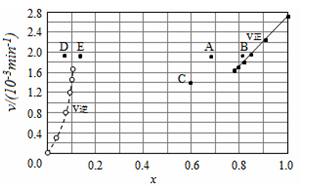
A���������¶ȵ�ijһ�¶ȣ��ٴδﵽƽ��ʱ����Ӧ����ֱܷ���A��E
B�����ٴγ���a mol HI����ﵽƽ��ʱ����Ӧ��ĺ�����ֵ���䣬������ֵ����
C�����ı������������ѹǿ���ٴδﵽƽ��ʱ����Ӧ����ı�����ǰ��ͬ
D�����ı��������ʹ�ô������ٴδﵽƽ��ʱ����Ӧ����ı�����ǰ��ͬ