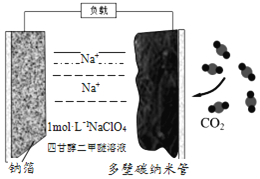
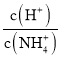��Ŀ����
����Ŀ����ʹ������к͵ζ����ⶨ���۰״ĺ�����(g/100 mL)��
��ʵ�鲽�裺
(1)��ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100 mL________(����������)�ж��ݣ�ҡ�ȼ��ô���״���Һ��
(2)����ʽ�ζ���ȡ����״���Һ20.00 mL����ƿ�У������еμ�2��________��ָʾ����
(3)��ȡʢװ0.1000 mol/L NaOH��Һ�ļ�ʽ�ζ��ܵij�ʼ���������Һ��λ������ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
![]()
(4)�ζ�����______________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��ʵ���¼
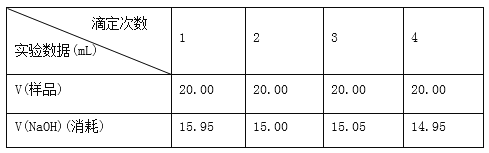
�����ݴ��������ۣ�
(1)����ȷ���ݴ������ɵ����۰״�������________g/100 mL(���������λ��Ч����)��
(2)��ͬѧ��ϸ�о��˸�Ʒ�ư״ı�ǩ���������л����б���������ΪʳƷ���Ӽ������������Ϸ���֤�����뱽�����Ʋ��ᷢ�����ӻ�����Ӧ���������һ���¶��µĴ����뱽�����______(��д���)��
a��pH b���е� c�����볣�� d���ܽ��
(3)�ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫ�����________(��д���)��
a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
c����ƿ�м������״���Һ���ټ�����ˮ
d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
���𰸡� ����ƿ ��̪ 0.70 ��Һ����ɫǡ�ñ�Ϊdz��ɫ�����ڰ�����ڲ���ɫ 4.500 c ab
�����������������I����1����Һ��ϡ�����ձ��н��У���Һ�Ķ����ڶ�Ӧ���������ƿ�н��У���2��ǿ��ζ�����ʱӦѡ����Է�Χ�ڱ�ɫ��ָʾ������3�����ݵζ��ܵĽṹ�뾫ȷ������������4���ζ��յ�ʱ��Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫ��III����1���������ݵĺ����������������ƽ�����ĵ�NaOH��Һ�������Ȼ����ݰ״���NaOH��Һ��Ӧ�Ĺ�ϵʽ����𣻣�2�����ݵ��볣ƽ�����ж����ǿ����Ȼ������ǿ������������������3������C�����⣩=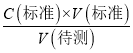 ���������
���������
������I����1����ȡ10.00mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�ж��ݣ�ҡ�ȼ��ô���״���Һ����2��ʳ����NaOH��Ӧ������ǿ�������Σ���Һ�ʼ��ԣ�Ӧѡ����Է�Χ�ڱ�ɫ��ָʾ����̪����3���ζ���Һ��Ķ���0.70mL����4��NaOH�ζ�ʳ���յ�Ϊ����Һ����ɫǡ�ñ�Ϊ��ɫ�����ڰ�����ڲ���ɫ�� III����1����1�εζ�������Դ����쳣ֵ��Ӧ��ȥ��3�����ĵ�NaOH��Һ�����Ϊ��15.00mL��15.05mL��14.95mL����NaOH��Һ�������ƽ��ֵΪ15.00mL��
��20mLϡ�ͺ�İ״���Ʒ����CH3COOOHXg����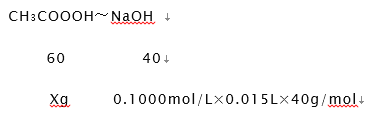
c�����۰״ף�=![]() g/100 mL����2�����ݵ��볣���Ĵ�С�ж�����ǿ���������жϷ�Ӧ�ܷ���������ѡ����ж����ǿ������ѡc����3��a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ����ҺŨ�Ƚ��ͣ����V������ƫ����C�����⣩=
g/100 mL����2�����ݵ��볣���Ĵ�С�ж�����ǿ���������жϷ�Ӧ�ܷ���������ѡ����ж����ǿ������ѡc����3��a����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ����ҺŨ�Ƚ��ͣ����V������ƫ����C�����⣩=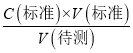 ��������֪C�����⣩ƫ��a��ȷ��b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����C�����⣩=
��������֪C�����⣩ƫ��a��ȷ��b����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫ����C�����⣩=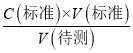 ��������֪C�����⣩ƫ��b��ȷ��c����ƿ�м������״���Һ���ټ�����ˮ����V��������Ӱ�죬����C�����⣩=
��������֪C�����⣩ƫ��b��ȷ��c����ƿ�м������״���Һ���ټ�����ˮ����V��������Ӱ�죬����C�����⣩=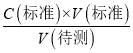 ��������֪C�����⣩���䣬��c����d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��������Һ���ʵ���ƫС�����V������ƫС������C�����⣩=
��������֪C�����⣩���䣬��c����d����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��������Һ���ʵ���ƫС�����V������ƫС������C�����⣩=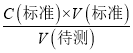 ��������֪C�����⣩ƫС����d����
��������֪C�����⣩ƫС����d����

����Ŀ���Ȼ���ͭ(CuCl)�������л��ϳɹ�ҵ�еĴ������ڳ�ʪ��������ˮ��������������ֽ⣬��ɺ�ɫ��CuCl�����ڴ���ˮ��������������Ũ�Ƚϴ����ϵ����ͼ�ǹ�ҵ��������ӡˢ��·�ķ�Һ����Fe3+��Cu2+��Fe2+��Cl-��
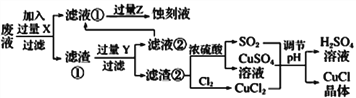
����������Ϣ�ش��������⣺
��1��д�������������������ʵ����ƣ�____________��________________��
��2��д������CuCl�Ļ�ѧ����ʽ��________________________________��
��3����CuCl�����ɹ����������ϲ���Ҫ����SO2���壬��ϻ�ѧ����ʽ�ͱ�Ҫ������˵������________________________________________________��ʵ��������SO2Ҫ�ʵ�������ԭ�������___����һ�㼴�ɣ���
��4���õ�CuC1�������Ҫ����ˮϴ���Ҵ�ϴ���������������Ҵ�ϴ�ӵ�������________________��
��5��ʵ��̽��pH��CuCl���ʵ�Ӱ�����±���ʾ��
pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
CuCl���� | 70 | 90 | 82 | 78 | 75 | 72 | 70 |
����CuCl�������pHΪ_________����pH�ϴ�ʱCuCl���ʱ��ԭ����________________��
��6���Ȼ���ͭ�Ķ���������
�ٳ�ȡ��Ʒ0.25g������FeCl3��Һ��250mL��ƿ�У�����ܽ⡣
����0.10molL-1���������Һ�ζ�����֪��CuCl+FeCl3�TCuCl2+FeCl2��Fe2++Ce4+�TFe3++Ce3+������ƽ��ʵ�������£�ƽ��ʵ�������ܳ���1%����
ƽ��ʵ����� | 1 | 2 | 3 |
0.25g��Ʒ�������������Һ�������mL�� | 24.35 | 24.05 | 23.95 | /tr>
����Ʒ��CuCl�Ĵ���Ϊ_______________�����������λ��Ч���֣���