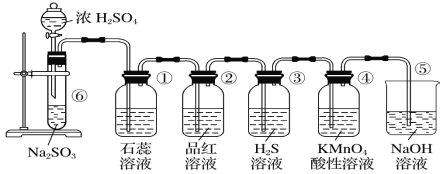
��Ŀ����
����Ŀ��I. �ϳ�����CO+H2���㷺���ںϳ��л����ҵ�ϳ�������Ȼ����ˮ������Ӧ�ȷ�������ȡ�ϳ�����
��1����֪��5.6L(�����)CH4��ˮ������ȫ��Ӧ������51.5KJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ_______________________________________________��
��2����150��ʱ2L���ܱ������У���2 mol CH4��2 mol H2O(g)��ϣ�����15min�ﵽƽ��,��ʱCH4��ת����Ϊ60%���ش��������⣺
�ٴӷ�Ӧ��ʼ��ƽ�⣬�������ı仯������ʾ�÷�Ӧ����v(H2)=____________��
���ڸ��¶��£�����÷�Ӧ��ƽ�ⳣ��K��________________________(������λС��)��
������ѡ�����ܱ�ʾ�÷�Ӧ�Ѵﵽƽ��״̬����__________________________
A.v(H2)����3v (CO)�� B.�ܱ������л��������ܶȲ���
C.�ܱ���������ѹǿ���� D.C (CH4) = C (CO)
��3���ϳ����е�����Ҳ���ںϳɰ�����N2 + 3H2![]() 2NH3�������¶Ⱥ�������䣬 �ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����___________��
2NH3�������¶Ⱥ�������䣬 �ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����___________��
�� �� | ��� | ��ʼ���� | ƽ��ʱNH3�����ʵ��� | ƽ��ʱN2�� ������� | ��Ӧ��ʼʱ������ | ƽ��ʱ������ѹǿ |
�� | 1L | 1molN2+3molH2 | 1.6mol | ���� | ���� | P�� |
�� | 1L | 2molN2+6molH2 | n1 mol | ���� | ���� | P�� |
�� | 2L | 2molN2+6molH2 | n2 mol | ���� | ���� | P�� |
A��n1=n2=3.2 B������=���������� C������������������ D��P����P��=P��
II.��1�������£���x mol��L��1��ˮ�м���������y mol��L��1����û����ҺMǡ�������ԡ�
��M��Һ����������Ũ���ɴ�С��˳��Ϊ_________________��
�ڳ����£�NH3��H2O�ĵ��볣��K=_____(�ú�x��y�Ĵ���ʽ��ʾ��������Һ���ǰ�������仯)��
��2�����õ�ⷨ�������¿�����ϡ����NO(O2Ũ��ԼΪNOŨ�ȵ�10��)��װ��ʾ��ͼ���£��������ʿɴ���O2��
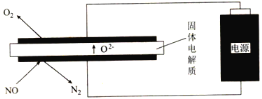
�����ĵ缫��ӦʽΪ___________________________��
������һ������NO�����ĵĵ���ԶԶ�������ۼ����������ܵ�ԭ����(��������������)________��
���𰸡�CH4��g��+H2O��g��=CO��g��+3H2��g��![]()
![]() 21.87 AC BD
21.87 AC BD ![]()
![]() 2NO+4e-=N2+2O2- ������������ӦO2+4e-=2O2-
2NO+4e-=N2+2O2- ������������ӦO2+4e-=2O2-
��������
I.(1)����£�5.6LCH4���ʵ���Ϊ��![]() =0.25mol������51.5kJ����������1mol���鷴Ӧ��������=51.5kJ��
=0.25mol������51.5kJ����������1mol���鷴Ӧ��������=51.5kJ��![]() =206kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+H2O(g)=CO(g)+3H2(g)��H=+206 kJ/mol��
=206kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+H2O(g)=CO(g)+3H2(g)��H=+206 kJ/mol��
(2)��150��ʱ2L���ܱ������У���2mol CH4��2mol H2O(g)��ϣ�����15min�ﵽƽ�⣬��ʱCH4��ת����Ϊ60%����
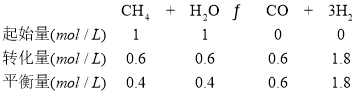
�ٴӷ�Ӧ��ʼ��ƽ�⣬�������ı仯������ʾ�÷�Ӧ����v(H2)=![]() =0.12molL-1min-1��
=0.12molL-1min-1��
�ڽ�Ϣټ���õ���ƽ��Ũ�ȣ�����õ��÷�Ӧ��ƽ�ⳣ��K=(1.83��0.6)/(0.4��0.4)=21.87��
��A��v��(H2)��3v��(CO)��˵�����淴Ӧ������ͬ����Ӧ�ﵽƽ��״̬����A��ȷ��
B���ܱ������л�������������������䣬�ܶ�ʼ�ղ��䣬����˵����Ӧ�ﵽƽ��״̬����B����
C����Ӧǰ���������ʵ������ӣ�����ѹǿ֮�ȵ����������ʵ���֮�ȣ��ܱ���������ѹǿ���䣬˵����Ӧ�ﵽƽ��״̬����C��ȷ��
D��Ũ�ȹ�ϵ������������ʼ���йأ�c(CH4)=c(CO)����˵����Ӧ�ﵽƽ��״̬����D����
�ʴ�Ϊ��AC��
(3)A���ͱ�Ϊ��Чƽ�⣬��n2=1.6mol�����������ȣ��൱������ѹǿ��ƽ�����������ƶ�����n1��3.2����A����
B���ͱ��ﵽƽ��״̬Ϊ��ͬƽ��״̬���������������ͬ�����൱�ڼ�ƽ��״̬�ټ���1mol������3mol����������ѹǿƽ��������У��������������С���ռ�=�ձ������ң���B��ȷ��
C���������з�Ӧ��Ũ�ȴ��ڼͱ�����Ӧ���ʴͱ���ʼŨ����ͬ��Ӧ������ͬ����C����
D����������Ũ���Ǽ�2������ѹǿ���ڼף��ͱ�Ϊ��Чƽ�⣬ѹǿ��ͬ���õ�P����P��=P������D��ȷ��
�ʴ�Ϊ��BD��
II.(1)�ٸ��ݵ���غ㣬c(NH4+)+c(H+)=c(OH-)+2c(SO42-)����Ϻ���Һ�����ԣ���c(NH4+)=2c(SO42-)����![]() ��
��
��x molL-1��ˮ�м���������y molL-1����û����ҺMǡ�������ԣ���c(NH4+)=2c(SO42-)=2��![]() molL-1=ymolL-1����Ϻ��������غ�c(NH3H2O)+c(NH4+)=0.5x molL-1����c(NH3H2O)=(0.5x-y)molL-1��K=c(NH4+)c(OH-)/c(NH3H2O)=y��1��10-7/(0.5x-y)=2y��10-7/(x-2y)��
molL-1=ymolL-1����Ϻ��������غ�c(NH3H2O)+c(NH4+)=0.5x molL-1����c(NH3H2O)=(0.5x-y)molL-1��K=c(NH4+)c(OH-)/c(NH3H2O)=y��1��10-7/(0.5x-y)=2y��10-7/(x-2y)��
(2)��������NO�õ���������N2������غ�ԭ����缫����ʽΪ2NO+4e-=N2+2O2-��
������һ������NO�����ĵĵ���ԶԶ�������ۼ����������ܴ��ڸ���Ӧ��O2Ũ��ԼΪNOŨ�ȵ�10���������õ���������O2-���缫����ʽΪ��O2+4e-=2O2-��