��Ŀ����
6�������Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����һ�ֻ�ѧԪ�أ�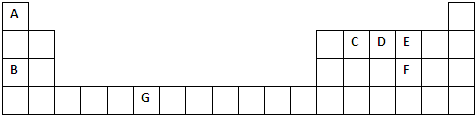
�Իش��������⣺
��1��C��һ����̬�⻯�����ˮ���Ĵ���������⻯������ЦҼ���м�����Ŀ֮��Ϊ5��1��
��2��C��D��E����Ԫ�ص�һ�������ɴ�С��˳��ΪN��O��C����Ԫ�ط��ţ������е縺��������O����Ԫ�ط��ţ���
��3��д��B��E�γɵĻ�����B2E2�ĵ���ʽ
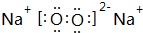 ��ָ���û������еĻ�ѧ�����Ӽ����Ǽ��Թ��ۼ���
��ָ���û������еĻ�ѧ�����Ӽ����Ǽ��Թ��ۼ�����4���ȽϷе㣺A2E����A2F������ڡ�����С�ڡ�����ԭ���ǣ�H2O���Ӽ���������H2S���Ӽ䲻���γ������
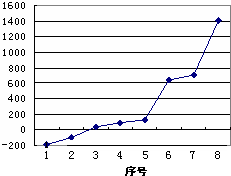
��5����������8��Ԫ�ذ������۵㣨�棩�ߵ͵�˳����ͼ��������š�8������Si����Ԫ�ط��ţ���
 ��6����д��Ԫ��G�Ļ�̬ԭ�ӵ����Ų�ʽ1s22s22p63s23p63d54s1����CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln��H2O��6-n]x+��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
��6����д��Ԫ��G�Ļ�̬ԭ�ӵ����Ų�ʽ1s22s22p63s23p63d54s1����CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln��H2O��6-n]x+��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��[CrCln��H20��6-n]x++xR-H��Rx[CrCln��H2O��6-n]+xH+
����������H+���к͵ζ����������x��n��ȷ�������ӵ���ɣ� ����0.0015mol[CrCl��H2O��6-n]x+����Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200mol•L-1 NaOH��Һ25.00ml���������ӵĻ�ѧʽΪ[CrCl��H2O��5]2+��
���� ��Ԫ�����ڱ��ṹ��֪��AΪHԪ�ء�BΪNaԪ�ء�CΪ̼Ԫ�ء�DΪNԪ�ء�EΪOԪ�ء�FΪSԪ�ء�GΪCrԪ�أ�
��1��C��һ����̬�⻯�����ˮ���Ĵ���������⻯��ΪCH2=CH2������Ϊ�Ҽ���˫������1���Ҽ���1���м���
��2��ͬ����������ҵ�һ�����ܳ��������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�ͬ������ԭ����������縺������
��3��������B2E2ΪNa2O2�����������������ӻ��������ʽ����Ҫ�����������������ɣ����������д������Ӽ��ͷǼ��Թ��ۼ���
��4��ˮ���������������������������ڷ��»����������۷е���������Ӽ�û�����������ˮ�ķе������ķе�ߣ�
��5�����������У��赥������ԭ�Ӿ��壬���۷е���ߣ�
��6��GΪCrԪ�أ�ԭ�Ӻ�����24�����ӣ����ݹ���ԭ�������ع���������д��������Ų���
�кͷ�����Ӧ��H++OH-=H2O�����к����ɵ�H+��Ҫ��NaOH��Һ�ɵó�H+���ʵ��������������x��[CrCln��H2O��6-n]x+��Cr�Ļ��ϼ�Ϊ+3�ۣ����ϼ۴����͵�������������ɣ��ݴ˼���n��ֵ������ȷ���������ӻ�ѧʽ��
��� �⣺��Ԫ�����ڱ��ṹ��֪��AΪHԪ�ء�BΪNaԪ�ء�CΪ̼Ԫ�ء�DΪNԪ�ء�EΪOԪ�ء�FΪSԪ�ء�GΪCrԪ�أ�
��1��C��һ����̬�⻯�����ˮ���Ĵ���������⻯��ΪCH2=CH2�������к���4��C-H������1��C=C˫���������ǦҼ���˫������1���Ҽ���1���м����Ҽ���м�����Ŀ֮��Ϊ5��1���ʴ�Ϊ��5��1��
��2��CΪCԪ�ء�DΪNԪ�ء�EΪOԪ�أ�ͬ����������ҵ�һ�����ܳ��������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�����ܴ�СΪ��N��O��C��ͬ������ԭ����������縺�����ʵ縺�Դ�СΪ��O��N��C���縺������ΪOԪ�أ��ʴ�Ϊ��N��O��C��O��
��3��BΪNaԪ�ء�EΪOԪ�أ�B��E�γɵĻ�����B2E2ΪNa2O2�����������������ӻ�����������Ƶĵ���ʽΪ�� �����������к������Ӽ��ͷǼ��Թ��ۼ���
�����������к������Ӽ��ͷǼ��Թ��ۼ���
�ʴ�Ϊ�� �����Ӽ����Ǽ��Թ��ۼ���
�����Ӽ����Ǽ��Թ��ۼ���
��4��ˮ����֮��������������ˮ����֮����������ǿ����H2S���Ӽ䲻���γ��������е㣺H2O��H2S��
�ʴ�Ϊ�����ڣ�H2O���Ӽ���������H2S���Ӽ䲻���γ������
��5����������8��Ԫ�صĵ����У�ֻ��SiΪԭ�Ӿ��壬�۷е������ͼ��֪��š�8��������ΪSi��
�ʴ�Ϊ��Si��
��6��GΪCrԪ�أ�ԭ�Ӻ�����24�����ӣ����Ժ�������Ų�ʽΪ��1s22s22p63s23p63d54s1��
�к����ɵ�H+��Ũ��Ϊ0.1200mol/L����������Һ25.00mL����H++OH-=H2O�����Եó�H+�����ʵ���Ϊ0.12mol/L��25.00��10-3L=0.003mol������x=$\frac{0.003mol}{0.0015mol}$=2��[CrCln��H2O��6-n]x+��Cr�Ļ��ϼ�Ϊ+3�ۣ�����3-n=2�����n=1�����������ӵĻ�ѧʽΪ[CrCl��H2O��5]2+��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��[CrCl��H2O��5]2+��
���� ���⿼��λ�ýṹ�����ʵĹ�ϵ����Ŀ�Ѷ��еȣ�ע��ͬ����Ԫ�صĵ�һ�������쳣�������6������Ŀ���ϸߣ�����ʹѧ������η��У���֪ʶ��ŵ�ͣ����÷���ʽ�����ϼ������ӵ�ɹ�ϵ���ɽ��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�| A�� | ��������һ�ֺϽ� | |
| B�� | ͨ���Ͻ��Ӳ�ȱȲ��ϴ����Ľ��� | |
| C�� | �Ͻ������ֽ����ۺ϶��ɵľ��н������Ե����� | |
| D�� | һ���˵�Ͻ���۵�����ĸ��ɷֽ������۵㶼�� |
| A�� | ǿ�ᡢǿ�����������ǿ����ʣ����ᡢ��������������� | |
| B�� | ���е����ӻ����ﶼ��ǿ����ʣ����еĹ��ۻ����ﶼ��������� | |
| C�� | ǿ�������Һ�ĵ�������һ�������������Һ�ĵ�������ǿ | |
| D�� | CO2��ˮ��Һ�ܵ��磬����CO2�ǵ���� |
| A�� | x+25 | B�� | x+2 | C�� | x+12 | D�� | x+26 |
| A�� | Fe�����ᷴӦ��H2��2Fe+6H+�T2Fe3++3H2�� | |
| B�� | ��Mg��OH��2��Һ�е���FeCl3��Һ��3Mg��OH��2+2Fe3+�T2Fe��������3+3Mg2+ | |
| C�� | ��AlCl3�Ͱ�ˮ�Ʊ�Al��OH��3��Al3++3OH-�TAl��OH��3�� | |
| D�� | ��ʯī�缫��ⱥ��ʳ��ˮ��2H++2Cl-$\frac{\underline{\;���\;}}{\;}$Cl2��+H2�� |
| A�� | �л���CH2=CHCH��CH3��CH2CH3�������ӳɺ�IJ����һ��ȡ������3�� | |
| B�� | 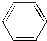 ��ʾ���ķ��ӽṹ�����к���̼̼˫������˱������ʸ���ϩ��ͬ ��ʾ���ķ��ӽṹ�����к���̼̼˫������˱������ʸ���ϩ��ͬ | |
| C�� | ��ϩʹ��ˮ��ɫ�ͱ�����ˮ�����ˮ���Ϊ��ɫԭ����ͬ | |
| D�� | CH2Cl2�Ǵ����˵�����������������νṹ������ƽ�������νṹ |
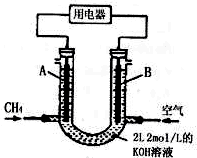 �縡ѡ���۷��ǹ�ҵ�ϲ��õ�һ����ˮ����������������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe��OH��3������Fe��OH��3�������ԣ�������������������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ����Ʋ���������㣬�����˸�ѡ���������ã�ij����С���õ縡ѡ���۷�������ˮ�����װ��ʾ��ͼ����ͼ��ʾ��
�縡ѡ���۷��ǹ�ҵ�ϲ��õ�һ����ˮ����������������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe��OH��3������Fe��OH��3�������ԣ�������������������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ����Ʋ���������㣬�����˸�ѡ���������ã�ij����С���õ縡ѡ���۷�������ˮ�����װ��ʾ��ͼ����ͼ��ʾ��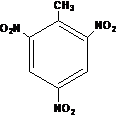 ��
��