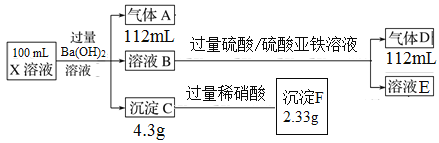
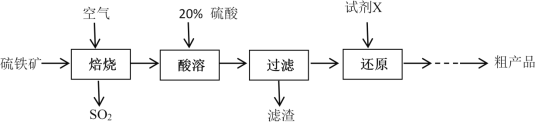
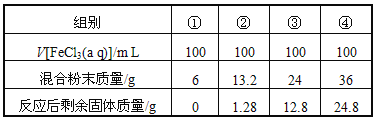
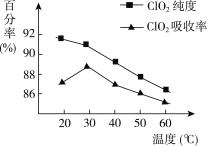
��Ŀ����
����Ŀ��ij����С��ͬѧ������CuO��NH3�ķ�Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ���Ƶ�ʵ��װ����ͼ��ʾ(�г�װ����ʡ��)��
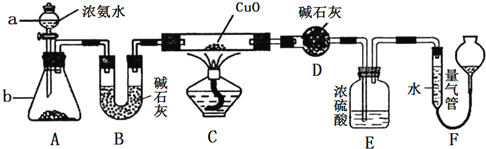
��ش���������:
��1������a������Ϊ______________������b��ʢװ���Լ�Ϊ__________________(������)��
��2��ʵ���й۲쵽װ��C�к�ɫCuO��ĩ��Ϊ����ͭ�����������������嵥�ʲ���������ʵ������֤��NH3����____________�ԣ�д����Ӧ�Ļ�ѧ����ʽ:_________________________��
��3��װ��E��Ũ�����������_________________________________��
��4��ʵ�����������ø����D����mg��װ��F����������ΪnL(������Ϊ��״��)�������е������ԭ�Ӹ�����Ϊ___________(�ú�m��n�Ĵ���ʽ��ʾ)��
���𰸡� ��Һ©�� ��ʯ��(�������ƻ��������ƹ�����ʯ��) ��ԭ 3CuO+2NH3![]() 3Cu+3H2O+N2 ����δ��Ӧ��İ�������ֹװ��F�е�ˮ��������װ��D 9n/11.2m(��18n/22.4m)
3Cu+3H2O+N2 ����δ��Ӧ��İ�������ֹװ��F�е�ˮ��������װ��D 9n/11.2m(��18n/22.4m)
����������������1������װ�����������ش𣬸��ݰ������Ʊ�ԭ�����
��2��C�к�ɫCuO��ĩ��Ϊ��ɫ���壬����������ɫ��ζ�����壬˵������ͭ��������������������ͭ��������ˮ��
��3��Eװ����Ũ�������������װ��ͼ�����ж�������Ũ�������չ���������ͬʱ����Fװ���е�ˮ��������D��
��4�������D����mgΪ��Ӧ���ɵ�ˮ��װ��F�����������ΪnLΪ��Ӧ���ɵĵ���������Ԫ���غ����õ���
��⣺��1��װ��������aΪ��Һ©��������b�����÷�Һ©���е���İ�ˮʹ��ƿ�еĹ����ܽ���Ȼ�����ѧ��Ӧ�ȴٽ�һˮ�ϰ��ֽ����ɰ������������ƹ��塢�����ƹ��塢��ʯ�ҹ���������㣻
��2��ʵ���й۲쵽װ��C�к�ɫCuO��ĩ��Ϊ��ɫ���壬����������ɫ��ζ�����壬˵������������ͭ��Ӧ����ͭ�͵�����ˮ������������ͭ�������ֻ�ԭ�ԣ����ԭ���غ���ƽд���÷�Ӧ�Ļ�ѧ����ʽΪ3CuO+2NH3![]() 3Cu+3H2O+N2��
3Cu+3H2O+N2��
��3���������̷�����Ũ���������չ����İ�������ֹF��ˮ��������DӰ��ʵ��Ч����
��4������ø����D����mgΪˮ�����ʵ���Ϊ![]() ��װ��F�����������ΪnL��������ɱ�״����ΪN2�����ʵ���Ϊ
��װ��F�����������ΪnL��������ɱ�״����ΪN2�����ʵ���Ϊ![]() ������Ԫ���غ�õ���ԭ�Ӻ���ԭ�����ʵ���֮�ȣ�
������Ԫ���غ�õ���ԭ�Ӻ���ԭ�����ʵ���֮�ȣ�![]() �������е������ԭ�Ӹ�����Ϊ
�������е������ԭ�Ӹ�����Ϊ![]() ��
��