��Ŀ����
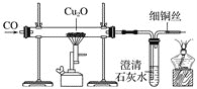
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸����)
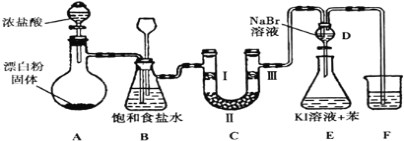
(1)װ��A�������ķ���װ�ã�Ũ�������Ư�۵���Ч�ɷַ�Ӧ������������д����Ӧ��Ӧ�Ļ�ѧ����ʽ��__________________________________��װ��A��������װƯ�۵�����������____________��
(2)װ��B�б���ʳ��ˮ��������___________________________��ͬʱװ��BҲ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д��C�з�������ʱB�е�����_________ ��
(3)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η������ʵ����Ӧ��___________(����ĸ���)��
��� | �� | �� | �� |
a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
b | �������ɫ���� | ��ˮ����ͭ | ʪ�����ɫ���� |
c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
(4)���װ��D��E��Ŀ����֤������ǿ��˳��Cl2 >Br2 >I2����Ӧһ��ʱ���������װ��D��������Һ����װ��E�У����۲쵽��������_____________________��������______(������������������)˵���嵥�ʵ�������ǿ�ڵ⣬ԭ����________________��
(5)װ��F��������___________�����ձ��е���Һ����ѡ�������е�____________(����ĸ���)��
a������NaOH��Һ b������NaCl��Һ
c������Na2SO3��Һ d������Na2CO3��Һ��
���𰸡�Ca(ClO)2+4HCl(Ũ)=CaCl2+2Cl2��+2H2O ������ƿ ��ȥCl2�е�HCl B�г���©����Һ���������γ�ˮ�� d E����Һ��Ϊ���㣬�²�(CCl4��)Ϊ�Ϻ�ɫ ���� ������Cl2Ҳ�ɽ�I-����ΪI2 ���ն������������ֹ��Ⱦ���� b
��������
(1)��![]() ��Ũ���ᷴӦ����������
��Ũ���ᷴӦ����������
(2)������ʳ��ˮ������ϴ������C�ж�������B��ѹǿ������
(3)����֤�����Ƿ���Ư���ԣ��������ø�����ɫ������ʪ�����ɫ���������Ա�ʵ�飻
(4)�����ڱ��е��ܽ�ȱ���ˮ�д����ڱ���Ϊ�Ϻ�ɫ��������![]() Ҳ�ɽ�
Ҳ�ɽ�![]() ����Ϊ
����Ϊ![]() ��
��
(5)���������ж����壬Ҫ����β������������![]() ��Һ���������ƽ��ٲ�����ȫ����ʣ���������
��Һ���������ƽ��ٲ�����ȫ����ʣ���������
(1)Ư�۵���Ч�ɷ���![]() ������ǿ�����ԣ���Ũ���ᷴӦ�ķ���ʽΪ
������ǿ�����ԣ���Ũ���ᷴӦ�ķ���ʽΪ ![]() �ʴ�Ϊ��
�ʴ�Ϊ��![]() ��
��
(2)����Ũ������лӷ��ԣ���Aװ�ó����������к����Ȼ������壬����ͨ������ʳ��ˮ��ȥ����C�ж�������B��ѹǿ������ˮ����ѹ���Ӷ��γ�һ��ˮ�����ʴ�Ϊ����ȥ![]() �е�
�е�![]() ��B�г���©����Һ���������γ�ˮ����
��B�г���©����Һ���������γ�ˮ����
(3)Ҫ��֤�����Ƿ���Ư���ԣ�����������ɫ��������Ϊ��ȡ����������ˮ���������ɵ�![]() ��Ư���ԣ�����֤�����Ƿ��ܹ�ʹʪ�����ɫ������ɫ��Ȼ���ø�������ͨ���������ɫ����������������Ư���ԡ�������������ѡ����������Ӧ�ļ�ʯ�ң�U�ι�һ��ʢװ����������Ũ�����ʢװ��U �ι��У���ˮ����ͭ���Լ���ˮ�Ĵ��ڣ���������������������ѡ����ˮ�Ȼ��ƣ�������ȷ��ѡ����d���ʴ�Ϊ��d��
��Ư���ԣ�����֤�����Ƿ��ܹ�ʹʪ�����ɫ������ɫ��Ȼ���ø�������ͨ���������ɫ����������������Ư���ԡ�������������ѡ����������Ӧ�ļ�ʯ�ң�U�ι�һ��ʢװ����������Ũ�����ʢװ��U �ι��У���ˮ����ͭ���Լ���ˮ�Ĵ��ڣ���������������������ѡ����ˮ�Ȼ��ƣ�������ȷ��ѡ����d���ʴ�Ϊ��d��
(4)��ͼ��֪��D��ͨ�����������ɵ����壬����E�к��������˵��ʵ⣬��E����������Һ�ֲ㣬���ϲ㼴�������Ϻ�ɫ����������D�п����й�������������E�����ɵĵ��ʵⲻһ��������KI��Ӧ�û������ģ�Ҳ�����ǹ�����![]() Ҳ��
Ҳ��![]() ����Ϊ
����Ϊ![]() �� �ʲ���˵���嵥�ʵ�������һ���ȵ�ǿ�� �ʴ�Ϊ��E����Һ��Ϊ���㣬�ϲ�(����)Ϊ�Ϻ�ɫ�����ܣ�������
�� �ʲ���˵���嵥�ʵ�������һ���ȵ�ǿ�� �ʴ�Ϊ��E����Һ��Ϊ���㣬�ϲ�(����)Ϊ�Ϻ�ɫ�����ܣ�������![]() Ҳ�ɽ�
Ҳ�ɽ�![]() ����Ϊ
����Ϊ![]() ��
��
(5)�����ж�����Ⱦ����������Fװ�õ����������ն����������F�в�����ʢװ����![]() ��Һ����Ϊ
��Һ����Ϊ![]() �ܽ��С�����ղ����ף��ʴ�Ϊ�����ն������������ֹ��Ⱦ������b��
�ܽ��С�����ղ����ף��ʴ�Ϊ�����ն������������ֹ��Ⱦ������b��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���⻯����ָһ�������£�1mol�����ͻ��������ʱ�ų��������������ǻ���ϩ��![]() ����������ϩ��
����������ϩ��![]() ���ͱ����⻯�����ݡ�����˵����ȷ����
���ͱ����⻯�����ݡ�����˵����ȷ����
������ |
|
|
|
�⻯�� | -119��7 | -232��7 | -208��4 |
A.���⻯�����ݿ��Ʋ⣬������ϩ��![]() �ķ�Ӧ�����
�ķ�Ӧ�����
B.����ϩ��������ϩ�ͱ�����ͬ�Ĺ�����
C.���ֻ������л�����ϩ���ȶ�����ǿ
D.���������£�1mol![]() ת��Ϊ
ת��Ϊ![]() ʱ�ų�����
ʱ�ų�����