��Ŀ����
������ҹ�������������ŷŵķ�����ʯ����ȡ���Ტ����ˮ��ļ����о���óɹ�����������������ͼ��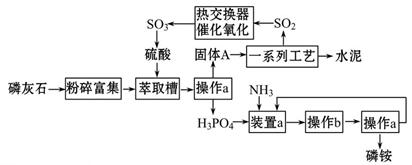
�ش��������⣺
��1������a��������___________��ʵ�����н��д˲����ķDz�����������Ʒ��________________����ʵ�����в���b��������______________________��
��2��װ��a������������ʽ�Σ����ǵĻ�ѧʽ�ֱ���_______________��
��3��������²����A��һ�����е����ʵĻ�ѧʽ��____________________(�ᾧˮ���ֲ�д)��
��4���Ƚ�������ʵ�����Ƚ�����װ�á���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�����Һ�Ƚ���ʱͨ��ʹ�õ�������________________________��
��5����������������β�����˺���N2��O2�⣬������SO2������SO3�������������ڲⶨ����β����SO2��������___________________��
| A��NaOH��Һ����̪��Һ | B��KMnO4��Һ��ϡ���� |
| C����ˮ��������Һ | D����ˮ����̪��Һ |
��1������ ����̨(����Ȧ)����ֽ ����Ũ������ȴ�ᾧ
��2��NH4H2PO4��(NH4)2HPO4
��3��CaSO4 ��4�������� ��5��B��C
���������������1����������a�õ�����A��H3PO4Һ�壬��������Һ��ķ����ǹ��ˣ����Բ���a�������ǹ��ˣ�������Ҫʢ��ҩƷ���ձ������˵�©�����������õIJ��������̶�©��������̨������Ȧ������ֽ�ȣ����ԷDz�����������Ʒ�У�����̨(����Ȧ)����ֽ������Һ����������ķ����ǣ�����Һ����Ũ������ȴ�ᾧ�ɵ���Ӧ���壬������ʵ�����в���b�������ǣ�����Ũ������ȴ�ᾧ��
��2��װ��a�������백��������Ӧ����������Ԫ�ᣬ�백����Ӧ�������ɣ�NH4��3PO4����NH4��2HPO4��NH4H2PO4�����Σ����У�NH4��2HPO4��NH4H2PO4������ʽ�Ρ�
��3������Ϣ��֪��������ŷŵķ�����ʯ����ȡ���ᣬ��ʯ����Ҫ�ɷ���Ca3��PO4��2������ȡ���������ᷢ����Ӧ�����������ɣ���������������Ʊ����̣�����A����һϵ�з�Ӧ����ȡH2SO4���ʹ���AӦΪCaSO4��
��4����ʵ������Һ���Ƚ�����װ���������ܣ��¿ڽ�ˮ�Ͽڴ�˭�������ˮ�������෴����ֽ����Ƚ��������Ը�����Ϊ�����ܡ�
��5��A��NaOH��Һ��SO2������SO3��������Ӧ��������SO2����ƫ�ߣ�����B������β����ֻ��SO2�ܱ�����KMnO4��Һ��������Һ��ɫ���Ϻ�ɫ��Ϊ��ɫ������KMnO4��Һ�������Ϸ���ʽ����SO2�ĺ�������ȷ��C������β����ֻ��SO2�ܱ���ˮ����SO2����Һ��ɫ����ɫ��Ϊ��ɫ�����ݵ�ˮ��Һ�������Ϸ���ʽ����SO2�ĺ�������ȷ��D����ˮNaOH��Һ��SO2������SO3��������Ӧ��������SO2����ƫ�ߣ�����
���㣺���⿼�黯ѧ�������̵ķ�����ʵ����������ͻ���������������жϼ�������

����ʵ�������ʹʵ����ƫ�͵���
| A��������ˮ��ʪ��pH��ֽ���ⶨ��ij����Һ��pH |
| B��������ƿ������Һ�����ݺ�ҡ��Һ���½����ټ�����ˮ���̶��������Ƶ���ҺŨ�� |
| C����������Ͳ�̶���ȡ��һ����Ũ���������Ƶ�0.1mol��L-1H2SO4��Һ��Ũ�� |
| D���ô���Һ��ϴ����ƿ�����к͵ζ����ⶨ�Ĵ���ҺŨ�� |
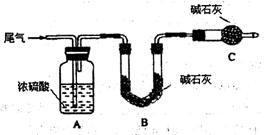
������(AlN)��һ�����������ϣ��㷺Ӧ���ڼ��ɵ�·����������ij�������к���̼���������е�һ�֣�����ͼI�е�һЩװ�������м��飬ʹ��������Ʒ��NaOH��Һ��Ӧ��AlN��NaOH��H2O=NaAlO2��NH3�������ݷ�Ӧ�������ɰ�����������ⶨ��Ʒ�еĵ�����������������������ʵ��������ȷ�����ʵijɷ�(ʵ���е���������Բ���)��
(1)ʵ���йز���Ϊ��a.����ƿ�з���������AlN��Ʒ��b.�ӷ�Һ©������ƿ�м��������ŨNaOH��Һ��c.����װ�õ������ԣ�d.�ⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ�� ��
(2)��ʵ����(ͼ��)���װ�������Եķ����ǣ� ��
(3)���ƿ�е��Լ�X��ѡ�� (��ѡ��ǰ�ı��)��
| A������ | B���ƾ� | C��ֲ���� | D��CCl4 |
(5)��ʵ���в����Ʒ������Ϊw g�����������Ϊa L(�����)������Ʒ��AlN����������Ϊ�� ��
(6)���˸���ͼ��װ�ý���ͬ��ʵ�飬ͨ���ⶨ�ձ����������ӵ�������ȷ����Ʒ��AlN����������������Ϊ�Ƿ���У� (����С������С�)��ԭ���� ��
Ba2����һ���ؽ������ӣ���һ�������С��������Na2S2O3��KI��K2Cr2O7���Լ��ⶨij������ˮ��Ba2�������ʵ���Ũ�ȡ�
(1)������ƿ��ʹ�÷����У����в�������ȷ����(����ĸ)________��
| A��ʹ������ƿǰ������Ƿ�©ˮ |
| B������ƿ��ˮϴ�������ô�����Һ��ϴ |
| C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ�����1��2 cm�����õι���εμ�����ˮ������ |
| D��������Һʱ����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������ˮ���ӽ�����1��2 cm�����õι���εμ�����ˮ������ |
(2)��������250 mL 0.100 mol��L��1�ı�Na2S2O3��Һ������Ҫ�IJ�����������Ͳ��250 mL����ƿ���������⣬����Ҫ________________��
(3)��ȷ��ȡNa2S2O3���������Ϊ________g��
(4)��ȡ��ˮ50.00 mL�������ʵ�����ȣ�����������K2Cr2O7��Һ���õ�BaCrO4������������ϴ�ӡ����˺���������ϡ�����ܽ⣬��ʱCrO42��ȫ��ת��ΪCr2O72�����ټ������KI��Һ���з�Ӧ��Ȼ���ڷ�ӦҺ�еμ�������Na2S2O3��Һ����Ӧ��ȫʱ������Na2S2O3��Һ36.00 mL����֪�йط�Ӧ�����ӷ���ʽΪ��Cr2O72����6I����14H��=2Cr3����3I2��7H2O����I2��2S2O32��=2I����S4O62������ù�����ˮ��Ba2�������ʵ���Ũ��Ϊ________��
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð״�����ƿ�У���ʵ������Ũ��Ϊc��mol/L�ı�NaOH��Һ������еζ���
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ���ԭ����������������������� ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
C����һ�εζ��õ���ƿδ��ϴ
D���ζ�����ʱ�����Ӷ���
��3�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ(��ʵ��ԭʼ������ʽ�����ػ���)��c������������ ������ ��
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��
��1��������������ȷ����______������ţ���
| A����Һ©�����ζ��ܺ�����ƿʹ��ǰ�������Ƿ�©ˮ |
| B������ˮ�����Һ©�����ټ������Ҵ�����������ã��ɴӵ�ˮ����ȡ�� |
| C���ྻ��������ʳ��ˮ�н���һ��ʱ�䣬�����������ݣ�˵�������������ⸯʴ |
| D����˿�������о���ȼ�գ��������䣬���ɺ�ɫ���� |
��2����ͭƬ��ϡ���ᷴӦ��ȡNO���壬��ͼװ�����ʺϵ���______������ţ���װ��B�е��Լ������______����װ�õ�������______________________��

 ��
��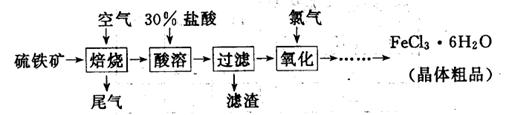
 6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .
6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .