��Ŀ����
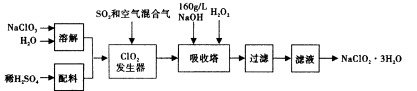
������п�к�����CuO��Fe3O4��SiO2�����ʡ���ҵ���Դ�����п��������п���壨ZnSO4��7H2O���Ĺ�����������ͼ��ʾ�� ��
��
��֪�������£���Һ�е�Fe3+��Zn2+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��6.5��9.7��
��ش��������⣺
��1�������۵������� ��
��2����������п�۵�����Ϊ ��
��3������30%H2O2��Ӧ�����ӷ���ʽ�� ��
��4����������Ca(OH)2������ҺpH���ٽ�Fe3+ˮ�⣬Fe3+ˮ�ⷴӦ��ƽ�ⳣ������ʽK���� ����Ca(OH)2���ܹ�����ԭ������ ����
����14�֣�
��1������Ũ������ȴ�ᾧ�����˸���(ÿ��1�֣���3��)��
��2��ʹ��Һ�е�Fe3+ת��ΪFe2+(2��)����ȥCu2+(2��)
��3��2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O (3��)
��4��c3(H+)/c(Fe3+) ��2�֣��� ��ֹ����Zn(OH)2��2�֣�
�������������ZnO��CuO��Fe3O4��SiO2���ڷ�Ӧ1��SiO2����H2SO4�����ˣ������٣���õ���Fe3+��Zn2+��Fe2+��H+��SO42-��Һ����������п��2Fe3++Zn= 2Fe2+ +Zn2+��Cu2++ Zn= Cu +Zn2+�����ˣ������ڣ���Zn2+��Fe2+��SO42-��Һ������30%H2O2ʹFe2+ת��ΪFe3+����������Ca(OH)2������ҺpH��ʹFe3+�������ɵ�Zn SO4��Һ��
��ZnSO4��Һ�õ�����п���壨ZnSO4��7H2O��Ӧ��������Ũ������ȴ�ᾧ�����˸��
п�ۿ��Է���2Fe3++Zn= 2Fe2+ +Zn2+��Cu2++ Zn= Cu +Zn2+�ķ�Ӧ��
���������Ϣ2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O��
��Fe3+ +3H2O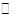 Fe (OH)3+3H+ K= c3(H+)/c(Fe3+)����Ca(OH)2����ʹ��Һ�ʼ���ʱZn2+����ȫ����Zn(OH)2������
Fe (OH)3+3H+ K= c3(H+)/c(Fe3+)����Ca(OH)2����ʹ��Һ�ʼ���ʱZn2+����ȫ����Zn(OH)2������
���㣺�����Թ�������Ϊ���壬����Ԫ�ؼ��仯��������ʼ�ʵ����������֪ʶ�����������������

 �Ķ��쳵ϵ�д�
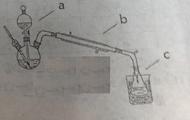
�Ķ��쳵ϵ�д�������ҹ�������������ŷŵķ�����ʯ����ȡ���Ტ����ˮ��ļ����о���óɹ�����������������ͼ��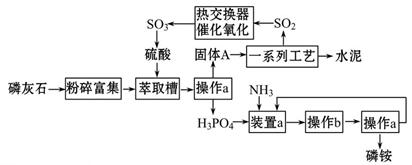
�ش��������⣺
��1������a��������___________��ʵ�����н��д˲����ķDz�����������Ʒ��________________����ʵ�����в���b��������______________________��
��2��װ��a������������ʽ�Σ����ǵĻ�ѧʽ�ֱ���_______________��
��3��������²����A��һ�����е����ʵĻ�ѧʽ��____________________(�ᾧˮ���ֲ�д)��
��4���Ƚ�������ʵ�����Ƚ�����װ�á���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�����Һ�Ƚ���ʱͨ��ʹ�õ�������________________________��
��5����������������β�����˺���N2��O2�⣬������SO2������SO3�������������ڲⶨ����β����SO2��������___________________��
| A��NaOH��Һ����̪��Һ | B��KMnO4��Һ��ϡ���� |
| C����ˮ��������Һ | D����ˮ����̪��Һ |
����a��e����ѧ��ѧʵ���г����ļ��ֶ���������
(a)��Ͳ (b)����ƿ (c)�ζ��� (d)������ƽ (e)�¶ȼ�
��1���ޡ�0���̶ȵ��� (��д���)��
��2�����в����������� (����ĸ)
| A����25mL��ʽ�ζ�����ȡ20.00mLNaHCO3 |
| B����������ƽȷ����10.20��̼���ƹ��� |
| C����100mL��Ͳ��ȡ3.2mLŨ���� |
| D������1 mol��L�C1������������Һ475mLѡ��500mL����ƿ |

��3��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������������Һ�����Ϊ mL��
��4��ͼ�ױ�ʾ10 mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1 mL������̶�AΪ4����Ͳ��Һ������Ϊ mL��ͼ�ұ�ʾ25 mL��ʽ�ζ�����ij��������D��E ֮��Ŀ̶Ȳ�Ϊ1 mL������̶�DΪ4�������ʽ�ζ�����Һ������Ķ���Ϊ mL��
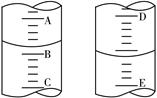
ͼ�� ͼ��
���в�����ʵ������Ԥ����ȷ����
| A��ʵ��I�����ã��²���Һ��ɫ���� |
| B��ʵ��� ���ձ����ȳ��ְ�ɫ���������ܽ� |
| C��ʵ��III������һ��ʱ���С�Թ����о������� |
| D��ʵ�����Ϊȷ��CuSO4���ɣ�����м�ˮ���۲���ɫ |
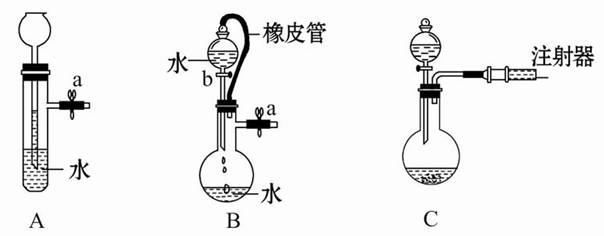

 ���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��

 ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol
ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol ��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ��
��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ �� N2+3Cu+3H2O�з�Ӧ��CuO��������H2O���������Բⶨͭ�Ľ������ԭ��������ʵ��װ�ã����ȼ��г�װ��δ���������¡�ʵ�鿪ʼʱ��Ӧ�ȵ�ȼ �����A������B�������ƾ��ƣ�c�м�ʯ�ҵ�����Ϊ ��
N2+3Cu+3H2O�з�Ӧ��CuO��������H2O���������Բⶨͭ�Ľ������ԭ��������ʵ��װ�ã����ȼ��г�װ��δ���������¡�ʵ�鿪ʼʱ��Ӧ�ȵ�ȼ �����A������B�������ƾ��ƣ�c�м�ʯ�ҵ�����Ϊ ��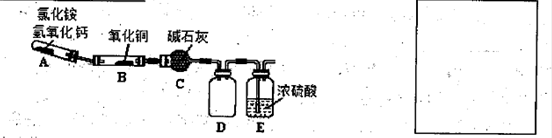
 ��Cl���ȣ�����ȡ�ϴ������Ȼ��ؾ��弰Һ�壬�������£�
��Cl���ȣ�����ȡ�ϴ������Ȼ��ؾ��弰Һ�壬�������£�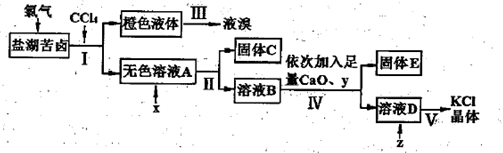
 CH3COOC2H5+H20
CH3COOC2H5+H20