��Ŀ����
��ѧ��һ����ʵ��Ϊ���Ŀ�ѧ����ѧʵ����ѧϰ̽���������ʵĻ�������֮һ��
��1�������й�������ȷ����__________����д��ţ�
a��ʹ��������ƽ�ĵ�һ�������ǽ��������������̶ȴ�
b�����˲��������У�Ϊ�ӿ�����ٶȿ��ò�������©���е���Һ���н���
c����Ũ��������ϡ��Һʱ������Ͳ�к�ϡ��Ҫ��ȴ��������ת�Ƶ�����ƿ��
d��������ƿ������Һʱ�����ݺ�ҡ��Һ���½����ټ�����ˮ���̶��ߴ���������ҺŨ��ƫ��
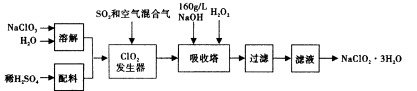
��2�������������壺H2��O2��NH3��SO2��NO2��NO������������ͼ��ʾװ�ý����ռ���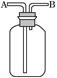
���������B�ڽ��룬���ռ���������_______________��
��������ƿ��ע��ˮ��������Ӧ�ô�______����д��A����B�����ڽ��룬�����ռ���������_________________________��
��1��a,d (2) �� ��B H2��O2��NO
���������������1��c������������Ͳ��ϡ�͡�d���ݺ�Ũ��Ϊ��ֵ�������ټ�ˮ����2����B�ڽ����ռ��ܶ�С�ڿ��������壬H2��NH3 . ����ˮ��Ӧ��B�ڽ���������ˮ���ռ���������H2��O2��NO��
���㣺�������ʵ���Ũ�ȵ����Ʋ������ռ�����ķ�����

 ��У����ϵ�д�
��У����ϵ�д����й���ʵ��ԭ��������������У�����ȷ����
| A���ж�������Ӧ�Ƿ���ȫ����ȡ��Ӧ��Ļ��Һ������ˮ�� |
| B����ѹ���˲��˹��˽�״���������̫С�ij��� |
| C���Ʊ���������茶��壬���������������������ʣ������Һ�弴ֹͣ���� |
| D����ȼ�Լ���ǿ�������Լ���ֿ����ã���Զ���Դ�������Ż�ʱ.����ϸɳ������� |
������ҹ�������������ŷŵķ�����ʯ����ȡ���Ტ����ˮ��ļ����о���óɹ�����������������ͼ��
�ش��������⣺
��1������a��������___________��ʵ�����н��д˲����ķDz�����������Ʒ��________________����ʵ�����в���b��������______________________��
��2��װ��a������������ʽ�Σ����ǵĻ�ѧʽ�ֱ���_______________��
��3��������²����A��һ�����е����ʵĻ�ѧʽ��____________________(�ᾧˮ���ֲ�д)��
��4���Ƚ�������ʵ�����Ƚ�����װ�á���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�����Һ�Ƚ���ʱͨ��ʹ�õ�������________________________��
��5����������������β�����˺���N2��O2�⣬������SO2������SO3�������������ڲⶨ����β����SO2��������___________________��
| A��NaOH��Һ����̪��Һ | B��KMnO4��Һ��ϡ���� |
| C����ˮ��������Һ | D����ˮ����̪��Һ |
��Ϊ��������ѧ���������ǿ�����ѧ����֪ʶ����������е�һЩ���⣬������ѧϰ����ҪĿ��֮һ������һ��ij��ѧʵ����ȤС�飬һ�����ˣ�����ʵ������ʦ�ṩ�Ļ���������ҩƷ�����й����˼�����ʳ��������Ʒ������������̽����
I. ��ͬѧ�ϼ���ɽ�����Զ�ʱ�ڼ���Ʒ������ɽ���ϳ´���ζ�������£����游ĸ������������Ǿ��ó����Ĵײ����ʱ��ζ��������������Ϻ�֪������Ϣ��
�ٴ����֣���������ƴס����ڵ��ϳ´ױ��뾭����ʱ������ŵô���ζ���г��϶����Ź�ҵ�����ˮ���ҵ����ƴס�
��������ұ�Ϊ���Ậ���������3.50 g/100mL�������ƴ��ұ���Ϊ1.50 g��3.50g/100mL��
������ʦ�İ����£��ⶨ�˳��й����ʳ���У���������ʵ���Ũ��Ϊ0.75mol/L��
��1���������ͬѧ����ӳ��й����ʳ���д��Ậ��Ϊ_______g/100mL������____________�ף�����족�����ơ���������ʾ������Ħ������Ϊ60g/mol��
��2����д�������뼦����(��Ҫ�ɷ�ΪCaCO3)��Ӧ�����ӷ���ʽ_______________________________��
II. ��ͼ������һ�л�ѧʵ����Ũ�����Լ���ǩ�ϵIJ������ݡ���ͬѧ���ø�Ũ������100mL1mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ�����ҩ�ף�����Ͳ����������ƽ���߲���������ش��������⣺
��1������ϡ����ʱ����ȱ�ٵ������� ��
��2�������㣬����100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ mL(����С�����һλ)��
��3���������Ƶ�ϡ������вⶨ��������Ũ��С��1mol/L����������ԭ������� ��
| A������ʱ��������ƿ�̶��� |
| B������ƿ��ʹ��ǰδ�����������������ˮ |
| C��ת����Һ��,δϴ���ձ��Ͳ����� |
| D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ��ټ�ˮ���̶��� |
��1�������������ļ����ĵ������Ƴ���Һ���ü����������Һ������һ������������������Һ���������Ϊ____________________��
��2��������ͬѧ���ƺõ�������Һ���뵽��������Һ�У����ֳ�����״�������������Ϊ_________��
IV. ��ͬѧ��ͼ�ⶨCO2����Է���������
�����ñ�ͬѧ�����ʣ�µļ����Ǻ���ͬѧ���ƺõ�ϡ������Һ�Ʊ�CO2��
�ڲ�ѯ����鼮�����������װ�ã�
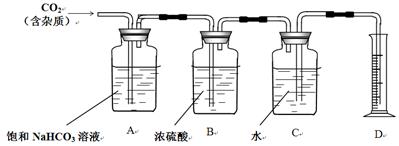
��1��Bװ���ڴ˴�____��Ҫ����д���С������ޡ��������ԭ��_________________________________��
��2��Aװ���еı���NaHCO3��Һ��������______________��
��3��ʵ��ǰ���Cװ��(��ˮ)����Ϊ50.00g��ʵ����Ϻ�Cװ�ã���ˮ������Ϊ40.02g��D����Ͳ����Ϊ10.0 mL����֪H2�ܶ�Ϊ0.09g/L(�������ݾ����ۺ�Ϊ�������ֵ)��������������ݣ�����CO2����Է�������Ϊ____________������С�����һλ����
��12�֣���������ƣ�Na2S2O3��5H2O�����������մ��ֳ�Ϊ������������������ˮ���������Ҵ��������ֽ⡣��ҵ�ϳ����������Ʒ�������Ʊ���ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ�����ͼ��ʵ������������Ϊ��
�ٿ�����Һ©����ʹ�����������£��ʵ����������У�ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3�Ļ����Һ�У�ͬʱ������Ž�����������
����������������ʧ��������Һ��pH�ӽ�
7ʱ��ֹͣͨ��SO2���塣
�۳������õ���Һ��ת�����������У�ˮԡ����
Ũ����ֱ����Һ������־�Ĥ��
����ȴ�ᾧ�����ˡ�ϴ�ӡ�
�ݽ������������У���40��45�����Ҹ���
50��60min��������
��ش��������⣺
��l������a�������� ��
��2���������������pHֵС��7������ʻ��½����������ӷ���ʽ����ԭ�� ��
��3��������в��ܽ���Һ�������ɵ�ԭ���� ����Ĥͨ������Һ������ֵ�ԭ���� ��
��4���������ϴ����������ƾ��������Լ��Ľṹʽ�� ��
��5��Ϊ�����ƵõIJ�Ʒ�Ĵ��ȣ���ʵ��С���ȡ5,0�˵IJ�Ʒ���Ƴ�250mL�����������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���25mL 0��0lmol��L-1 KIO3��Һ�������������KI���ữ���������з�Ӧ��5I��+IO3��+6H+=3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O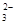 =2I��+S4O
=2I��+S4O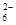 ������ɫ��ȥH����Ӳ���ɫʱ����ζ��յ㡣ʵ���������±���
������ɫ��ȥH����Ӳ���ɫʱ����ζ��յ㡣ʵ���������±���
| ʵ����� | 1 | 2 | 3 |
| Na2S2O3��Һ�����mL�� | 19.98 | 20.02 | 21.18 |
��ò�Ʒ�Ĵ�����____ ����ӵ������ζ������п������ʵ����ƫ�͵���____ ��
A���ζ���ĩ��Na2S2O3��Һ��ϴ B���ζ��յ�ʱ���Ӷ���
C����ƿ������ˮ��ϴ D���ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢������