��Ŀ����
20��ijѧ��������6.0mol?L-1��H2SO4��Һ1000mL��ʵ���������ֲ�ͬ�����ͬŨ�ȵ������480mL 0.5mol?L-1�������150mL 25%�����ᣨ��=1.18g?mL-1������������18mol?L-1�����ᣮ�����ֹ�������ƿ��250mL��500mL��1000mL����ʦҪ��Ѣ١�����������ȫ�����꣬����IJ����ɢ������䣮��ش��������⣺��1��ʵ����25%����������ʵ���Ũ��Ϊ3.0mol?L-1������1λС����
��2�����Ƹ�������ҺӦѡ������ƿ�Ĺ��Ϊ1000mL��
��3������ʱ����ͬѧ����˳�����£�������������D����������
A�����١�������Һȫ�����ձ��л�Ͼ��ȣ�
B������Ͳȷ��ȡ�����18mol?L-1��Ũ����295.0mL���ز����������������Һ�У����ò�����������ʹ���Ͼ��ȣ�
C������Ͼ��ȵ������ز�����ע����ѡ������ƿ�У�
D����������ˮϴ�ӱ��Ͳ�����2-3�Σ�ϴ��Һ��ע������ƿ�У�
E��������������ƿ�м��룬ֱ��Һ��ӽ��̶���1��2cm��
F�����ý�ͷ�μ�ˮ��ʹ��Һ�İ�Һ��ǡ����̶����У�
G��������ƿ�ǽ�����ҡ�ȣ�
��4�����ʡ�Բ���D��������ҺŨ���к�Ӱ�죺ƫС�����ƫ��ƫС������Ӱ�족��
��5�����в���Cǰ����ע�⽫ϡ�ͺ��������ȴ�����£�
���� ��1������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�
��2������������Һ���ѡ����ʵ�����ƿ��
��3��������Һϡ���������ʵ����ʵ������������ҪŨ���������
Ϊ��֤�������ʶ�ת�Ƶ�����ƿ��Ӧ���ձ��Ͳ���������ϴ�ӣ�����ϴ��Һ��ע������ƿ�У�
��4������C=$\frac{n}{V}$�������ʡ�Բ���D��������ҺŨ�ȵ�Ӱ�죻
��5������ƿΪ������������Һǰ����Һ�¶�Ӧ��Ϊ���£�
��� �⣺��1��25%�����ᣨ��=1.18g?mL-1�������ʵ���Ũ��Ϊ$\frac{1000��25%��1.18}{98}$=3.0mol/L���ʴ�Ϊ��3.0��
��2������6.0mol?L-1��H2SO4��Һ1000mL������Ӧѡ��1000mL��������ƿ���ʴ�Ϊ��1000��
��3����������6.0mol?L-1��H2SO4��Һ1000mL������ҪŨ�������ΪV��������Һϡ������������Һ�����ʵ�������ã�6.0mol?L-1��1L=0.5mol?L-1��
0.48L+3.0mol/L��0.15L+18mol?L-1��V�����V=0.295L����295��OmL��
Ϊ��֤�������ʶ�ת�Ƶ�����ƿ��Ӧ���ձ��Ͳ���������ϴ��2-3�Σ�����ϴ��Һ��ע������ƿ�У�
�ʴ�Ϊ��295.0����������ˮϴ�ӱ��Ͳ�����2-3�Σ�ϴ��Һ��ע������ƿ�У�
��4�����ʡ�Բ���D���������ʵ����ʵ���ƫС������C=$\frac{n}{V}$��֪��ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫС��
��5������ƿΪ������������Һǰ����Һ�¶�Ӧ��Ϊ���£��ʴ�Ϊ����ϡ�ͺ��������ȴ�����£�
���� ���⿼��������һ�����ʵ���Ũ�ȵ����Ƽ��㣬��ȷ����ԭ���ǽ���ؼ���������ѵ���������ƿ����ѡ����Ŀ�ѶȲ���

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�| A�� | 2-������������� | B�� | ��������ͬ���ṹ��ͬ���������� | ||
| C�� |  �� ��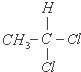 | D�� | 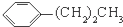 �� �� |
| A�� | 50 mL 1.5 mol•L-1��FeCl2��Һ | B�� | 100 mL 3 mol•L-1��NH4Cl��Һ | ||
| C�� | 75 mL 3 mol•L-1��KCl��Һ | D�� | 50 mL 2 mol•L-1��CaCl2��Һ |
 ����һ�������������MgSO4�Ļ����Һ����μ���Ba��OH��2��Һ����������������m �����Ba��OH��2�����ʵ���n֮��Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
����һ�������������MgSO4�Ļ����Һ����μ���Ba��OH��2��Һ����������������m �����Ba��OH��2�����ʵ���n֮��Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | O��aʱ�ķ�Ӧ�����ӷ���ʽΪ��Ba2++2OH-+SO42-+Mg2+�TBaSO4��+Mg��OH��2�� | |
| B�� | a��bʱ���������ӷ�ӦΪH++OH-=H2O | |
| C�� | ��a��b��c˵����Һ�н��OH-������ǿ��Ϊ��H+��Mg2+ | |
| D�� | ԭ�����Һ�� C��HCl����C��MgSO4��=4��1 |
 ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â����ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3���۵㡢�е��������£�
ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â����ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3���۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | - |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ�NaOH��Һ����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��3��ʵ�����ܽ��������������Լ��������˵��Լ���B������ţ���
A��Ũ���� B��ϡ����
C��ϡ���� D������������Һ��
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ����������������������ĺ�ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol•L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
��1��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪH++OH-�TH2O��
��2����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪNH4++OH-�TNH3•H2O����������˵����Һ��Al3+��Fe3+��H+���OH-��������NH4+ǿ�������ӷ��ţ���
��3��B��A�IJ�ֵΪ0.08mol��
��4��B���Ӧ�ij��������ʵ���Ϊ0.032mol��C���Ӧ������������Һ�����Ϊ7mL��
| A�� | ������۵��Ӳ�ȴ����������뵼����� | |
| B�� | ��������������������ԣ���������θ���кͼ� | |
| C�� | Ư���ڿ����в��ȶ���������Ư��ֽ�� | |
| D�� | �������ʺ���ɫ��������������ɫͿ�� |
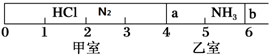
 ��1���Ӻ�����л���Һ����ȡ��ͻ����л��ܼ�����Ҫ��������ָ����������װ��ͼ�еĴ���֮����
��1���Ӻ�����л���Һ����ȡ��ͻ����л��ܼ�����Ҫ��������ָ����������װ��ͼ�еĴ���֮����