��Ŀ����
15����ͼ��ʾ��һ�ܱ���������Ħ�����ɻ�����������a��b�ֳɼס������ң���״���£��������г���NH3 0.4mol�������г���HCl��N2�Ļ�����壬��ֹʱ����λ����ͼ��ʾ����֪�ס��������������������Ϊ17.3g��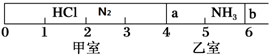
��1�����������������Ϊ24.1g��
��2��������HCl��N2�����ʵ���֮��Ϊ1��3��
��3��������aȥ������HCl��NH3��ַ�Ӧ����NH4Cl����������˷�Ӧ��������b��λ�ڿ̶ȡ�4�����������֣������ǹ������ʲ�����ѹǿ������ʱ��ϵ��ƽ��Ħ������25.25g/mol��
���� ��1���������NH3������m=nM�������������������������������������������
��2�����ݼ�������������ʵ�������������HCl�͵��������ʵ���֮�ȣ�
��3������ʣ����������ʵ�������ʣ��������ռ������Ӷ�ȷ��b��λ�ã�����M=$\frac{m}{n}$���㣮
��� �⣺��1�������г���NH3������������Ϊm=nM=0.4mol��17g/mol=6.8g����֪�ס��������������������Ϊ17.3g����������������Ϊ17.3g+6.8g=24.1g��
�ʴ�Ϊ��24.1g��
��2����ͬ�����£���������ʵ���֮�ȵ��������֮�ȣ���ͼ��֪�ס�����������������Ϊ2��1���������ʵ���֮��Ϊ2��1���������г���NH3 0.4mol�����Լ���������Ϊ0.8mol���������������Ϊ24.1g��
��HCl�����ʵ���Ϊx�����������ʵ���Ϊy��
���������ʵ����������з�����Ϊ��$\left\{\begin{array}{l}{x+y=0.8}\\{36.5x+28y=24.1}\end{array}\right.$�����x=0.2mol��y=0.6mol������HCl��N2�����ʵ���֮��Ϊ0.2mol��0.6mol=1��3��
�ʴ�Ϊ��1��3��
��3��������NH3�����ʵ���Ϊ0.4mol������HCl�����ʵ���Ϊ0.2mol�����Է�Ӧ����NH4Cl���壬ʣ��NH3�����ʵ���Ϊ0.2mol��ʣ������������ʵ���Ϊ0.8mol����ͬ�����£���������֮�ȵ��������ʵ���֮�ȣ���ʼʱ���������ʵ���Ϊ1.2mol�������b��6�����������������ʵ���Ϊ0.8mol�����Ի���b������������4��������ϵ��ƽ��Ħ������M=$\frac{m}{n}$=$\frac{0.2mol��17g/mol+0.6mol��28g/mol}{0.2mol+0.6mol}$=25.25g/mol��
�ʴ�Ϊ��4��25.25g/mol��
���� ���⿼�����ʵ�����Ũ�ȵļ��㣬�������ʵ���Ϊ���ĵĻ������㹫ʽ����ȷ��ͬ��������������֮�ȵ������ʵ���֮��Ϊ���Ĺؼ������ط�������������Ŀ��飬��Ŀ�Ѷ��еȣ�

| A�� | ���ó���Ϊ0.05 mol��BaSO4 | B�� | ���������SO2�����Ϊ0.448L | ||
| C�� | a L�����������ʵ���Ϊ0.04mol | D�� | a��ȡֵ��ΧΪ0.672��a��0.896 |
| A�� | ��λʱ��������amolX��ͬʱ����3amolY | |
| B�� | X��Y��Z�ķ�����֮��Ϊ1��3��2 | |
| C�� | Z������������Z�ķֽ�������� | |
| D�� | ��λʱ������3amolY��ͬʱ����3amolZ |
| A�� |  ��0.01 mol NaOH��0.01 mol Ba��OH��2�Ļ����Һ�л���ͨ��CO2 | |
| B�� |  KHCO3��Һ����μ���Ba��OH��2��Һ | |
| C�� | 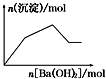 KAl��SO4��2��Һ����μ���Ba��OH��2��Һ | |
| D�� | 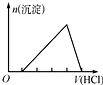 ���������������Ƶ�ƫ��������Һ�еμ����� |
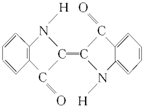
| A�� | ���������ڷ����廯���� | B�� | ���ķ���ʽ��C16H10N2O2 | ||
| C�� | ������ȫȼ�����ɶ�����̼��ˮ | D�� | ���Dz����͵��л��� |

 ����Ӧ�۵Ļ�ѧ����ʽΪ2CO2+2Na2O2=2Na2CO3+O2��
����Ӧ�۵Ļ�ѧ����ʽΪ2CO2+2Na2O2=2Na2CO3+O2��