ΧβΡΩΡΎ»ί
Β―ι “≈δ÷Τ500 mL 0.2 molΓΛLΘ≠1ΒΡNa2SO4»ή“ΚΘ§ Β―ι≤ΌΉς≤Ϋ÷η”–ΘΚ
AΘ°‘ΎΧλΤΫ…œ≥Τ≥ω14.2 gΝρΥαΡΤΙΧΧεΘ§Α―ΥϋΖ≈‘Ύ…’±≠÷–Θ§”Ο ΝΩΒΡ’τΝσΥ° ΙΥϋΆξ»Ϊ»ήΫβ≤Δά以 ÷Ν “Έ¬ΓΘ
BΘ°Α―÷ΤΒΟΒΡ»ή“Κ–Γ–ΡΒΊΉΣ“ΤΒΫ»ίΝΩΤΩ÷–ΓΘ
CΘ°ΦΧ–χœρ»ίΝΩΤΩ÷–Φ”’τΝσΥ°÷Ν“ΚΟφΨύΩΧΕ»œΏ1ΓΪ2 cm¥ΠΘ§ΗΡ”ΟΫΚΆΖΒΈΙή–Γ–ΡΒΈΦ”’τΝσΥ°÷Ν»ή“ΚΑΦ“ΚΟφΉνΒΆ¥Π”κΩΧΕ»œΏœύ«–ΓΘ
DΘ°”Ο…ΌΝΩ’τΝσΥ°œ¥Β”…’±≠ΚΆ≤ΘΝßΑτ2ΓΪ3¥ΈΘ§ΟΩ¥Έœ¥Β”ΒΡ“ΚΧεΕΦ–Γ–ΡΉΔ»κ»ίΝΩΤΩΘ§≤Δ«α«α’ώΒ¥ΓΘ
EΘ°ΫΪ»ίΝΩΤΩΤΩ»ϊ»ϊΫτΘ§≥δΖ÷“Γ‘»ΚσΉΑΤΩΓΘ
«κΧν–¥œ¬Ν–Ω’ΑΉΘΚ
Θ®1Θ©≤ΌΉς≤Ϋ÷ηΒΡ’ΐ»ΖΥ≥–ρΈΣ(Χν–ρΚ≈)____ _ΓΘ
Θ®2Θ©±Ψ Β―ι”ΟΒΫΒΡΜυ±Ψ“«Τς“―”–…’±≠ΓΔΧλΤΫ(¥χμά¬κΓΔΡςΉ”)ΓΔ≤ΘΝßΑτΘ§ΜΙ»±…ΌΒΡ“«Τς «________ΓΔ________ΓΘ
Θ®3Θ©œ¬Ν–«ιΩωΜα ΙΥυ≈δ»ή“Κ≈®Ε»ΤΪΗΏΒΡ «(Χν–ρΚ≈)______ __ΓΘ
AΘ°»ίΝΩΤΩ Ι”Ο«ΑΡΎ±Ύ’¥”–Υ°÷ι BΘ°ΟΜΫχ––…œ ωΒΡ≤ΌΉς≤Ϋ÷ηD
CΘ°Φ”’τΝσΥ° ±Θ§≤Μ…ς≥§ΙΐΝΥΩΧΕ»œΏ DΘ°μά¬κ…œ’¥”–‘”÷
Θ®1Θ©A B D C E Θ®2Θ© 500 mL »ίΝΩΤΩ ΒΈΙή Θ®3Θ©D
ΫβΈω ‘ΧβΖ÷ΈωΘΚΘ®1Θ©≈δ÷Τ“ΜΕ®ΧεΜΐΓΔ“ΜΕ®≈®Ε»ΒΡ»ή“ΚΒΡ≤Ϋ÷η «ΦΤΥψΓΔ≥ΤΝΩΘ®ΜρΝΩ»ΓΘ©ΓΔ»ήΫβΓΔ“Τ“ΚΓΔœ¥Β”ΓΔΕ®»ίΓΔ“Γ‘»ΓΘΙ ’ΐ»ΖΥ≥–ρ «A B D C EΓΘΘ®2Θ©±Ψ Β―ι”ΟΒΫΒΡΜυ±Ψ“«Τς“―”–…’±≠ΓΔΧλΤΫ(¥χμά¬κΓΔΡςΉ”)ΓΔ≤ΘΝßΑτΘ§ΜΙ»±…ΌΒΡ“«Τς «500 mL »ίΝΩΤΩ ΒΈΙήΓΘΘ®3Θ©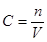 ΓΘ»τAΘ°»ίΝΩΤΩ Ι”Ο«ΑΡΎ±Ύ’¥”–Υ°÷ιΘ§‘ρΉνΚσΕ®»ί ±…ΌΦ”–©Υ°Θ§Ε‘»ή“ΚΒΡ≈®Ε»Έό”ΑœλΓΘ¥μΈσΓΘ»τBΘ°ΟΜΫχ––…œ ωΒΡ≤ΌΉς≤Ϋ÷ηDΘ§‘ρ”…”Ύ»ή÷ Φθ…ΌΘ§nΦθ–ΓΘ§Υυ“‘≈®Ε»ΤΪ–ΓΓΘ¥μΈσΓΘ»τCΘ°Φ”’τΝσΥ° ±Θ§≤Μ…ς≥§ΙΐΝΥΩΧΕ»œΏΘ§”…”Ύ»ή“ΚΒΡΧεΜΐΤΪ¥σΘ§ΒΦ÷¬≈®Ε»ΤΪΒΆΓΘ¥μΈσΓΘDΘ°»τμά¬κ…œ’¥”–‘”÷ Θ§‘ρ“‘¥Υμά¬κΈΣ±ξΉΦ≥ΤΝΩΒΡ»ή÷ ΒΡ÷ ΝΩΤΪ¥σΘ§nΤΪ¥σΘ§Υυ≈δ»ή“Κ≈®Ε»ΨΆΤΪ¥σΓΘ’ΐ»ΖΓΘ
ΓΘ»τAΘ°»ίΝΩΤΩ Ι”Ο«ΑΡΎ±Ύ’¥”–Υ°÷ιΘ§‘ρΉνΚσΕ®»ί ±…ΌΦ”–©Υ°Θ§Ε‘»ή“ΚΒΡ≈®Ε»Έό”ΑœλΓΘ¥μΈσΓΘ»τBΘ°ΟΜΫχ––…œ ωΒΡ≤ΌΉς≤Ϋ÷ηDΘ§‘ρ”…”Ύ»ή÷ Φθ…ΌΘ§nΦθ–ΓΘ§Υυ“‘≈®Ε»ΤΪ–ΓΓΘ¥μΈσΓΘ»τCΘ°Φ”’τΝσΥ° ±Θ§≤Μ…ς≥§ΙΐΝΥΩΧΕ»œΏΘ§”…”Ύ»ή“ΚΒΡΧεΜΐΤΪ¥σΘ§ΒΦ÷¬≈®Ε»ΤΪΒΆΓΘ¥μΈσΓΘDΘ°»τμά¬κ…œ’¥”–‘”÷ Θ§‘ρ“‘¥Υμά¬κΈΣ±ξΉΦ≥ΤΝΩΒΡ»ή÷ ΒΡ÷ ΝΩΤΪ¥σΘ§nΤΪ¥σΘ§Υυ≈δ»ή“Κ≈®Ε»ΨΆΤΪ¥σΓΘ’ΐ»ΖΓΘ
ΩΦΒψΘΚΩΦ≤ιΈο÷ ΒΡΝΩ≈®Ε»ΒΡ»ή“ΚΒΡ≈δ÷Τ≤Ϋ÷ηΓΔ4ΒΡ“«ΤςΦΑΈσ≤νΖ÷ΈωΒ»÷Σ ΕΓΘ

»Γ20 mL NaOH»ή“ΚΤΫΨυΖ÷≥…ΝΫΖίΘ§Ζ÷±πΖ≈»κAΓΔBΝΫ÷ß ‘Ιή÷–ΓΘœρAΓΔB÷–Ά®»κ≤ΜΒ»ΝΩΒΡCO2Θ§‘ΌΦΧ–χœρΝΫ»ή“Κ÷–÷πΒΈΦ”»κ0.1mol/LΒΡ―ΈΥαΘ§±ξΉΦΉ¥Ωωœ¬≤ζ…ζΒΡCO2ΤχΧεΧεΜΐ”κΥυΦ”ΒΡ―ΈΥα»ή“ΚΧεΜΐ÷°ΦδΒΡΙΊœΒ»γœ¬±μΥυ ΨΘΚ
| ―ΈΥαΧεΜΐΘ®ΒΞΈΜΘΚmLΘ© | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| A≤ζ…ζCO2ΒΡΧεΜΐ | 0 | 0 | 0 | 0 | 0 | 22.4 | 44.8 | 44.8 | 44.8 |
| B≤ζ…ζCO2ΒΡΧεΜΐ | 0 | 0 | 22.4 | 44.8 | 67.2 | 89.6 | x | x | x |
Θ®1Θ©…ΌΝΩCO2”κNaOH»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ Θ§
ΙΐΝΩCO2”κNaOH»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ΘΜ
Θ®2Θ© ‘ΙήA÷–Ά®»κCO2ΚσΥυΒΟ»ή“ΚΒΡ»ή÷ ΈΣ ΘΜ
Θ®3Θ©‘≠NaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ mol/LΘΜ
Θ®4Θ©ΒΈΦ”70mL―ΈΥα ±Θ§AΓΔB≤ζ…ζCO2ΒΡΧεΜΐΨυΈΣΉν¥σ÷ΒΘ§‘ρx= mLΓΘ
Χλ»ΜΦνΒΡΉι≥…Ω…“‘”Ο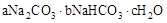 Θ®aΓΔbΓΔcΈΣ’ϊ ΐΘ©±μ ΨΓΘœ÷”–AΓΔBΝΫ÷÷≤ΜΆ§ΒΡΧλ»ΜΦν―υΤΖΘ§Ζ÷±πΫχ––»γœ¬ Β―ι“‘»ΖΕ®ΤδΜ·―ß ΫΓΘ
Θ®aΓΔbΓΔcΈΣ’ϊ ΐΘ©±μ ΨΓΘœ÷”–AΓΔBΝΫ÷÷≤ΜΆ§ΒΡΧλ»ΜΦν―υΤΖΘ§Ζ÷±πΫχ––»γœ¬ Β―ι“‘»ΖΕ®ΤδΜ·―ß ΫΓΘ
ΫΪ÷ ΝΩΈΣ31.0 gΒΡΧλ»ΜΦνA”Ύ300ΓφΦ”»»Ζ÷Ϋβ÷ΝΆξ»ΪΘ®300Γφ ±Na2CO3≤ΜΖ÷ΫβΘ©Θ§≤ζ…ζCO2 2.24 LΘ®±ξΉΦΉ¥ΩωΘ©ΚΆΥ°5.4 gΓΘ
Θ®1Θ©Χλ»ΜΦνAΒΡΜ·―ß Ϋ÷–ΘΚ ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ
ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ
ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ
ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ
“―÷ΣΘΚNa2CO3”κœΓ―ΈΥαΒΡΖ¥”ΠΖ÷œ¬Ν–ΝΫ≤ΫΫχ––ΘΚ
Na2CO3+HCl NaCl+NaHCO3 NaHCO3+HCl
NaCl+NaHCO3 NaHCO3+HCl NaCl+CO2Γϋ+H2O
NaCl+CO2Γϋ+H2O
ΫΪ÷ ΝΩΈΣ12.45 gΒΡΧλ»ΜΦνB»ή”ΎΥ°Θ§÷πΒΈΒΈΦ”Ρ≥≈®Ε»ΒΡœΓ―ΈΥαΘ§≤ζ…ζΤχΧεΒΡΧεΜΐ”κΦ”»κ―Έ
ΥαΒΡΧεΜΐΘ®±ξΉΦΉ¥ΩωΘ©ΒΡΙΊœΒ»γœ¬±μΥυ ΨΘΚ
| ―ΈΥαΧεΜΐΘ®mLΘ© | 20 | 40 | 60 | 80 |
| ≤ζ…ζΤχΧεΧεΜΐΘ®mLΘ© | 0 | 560 | 1680 | 2520 |
Θ®2Θ©”…±μ÷– ΐΨίΩ…ΆΤ≤βΦ”»κ50mL―ΈΥα ±Θ§≤ζ…ζΤχΧεΒΡΧεΜΐΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓmLΘ®±ξΉΦΉ¥ΩωΘ©ΘΜ―ΈΥαΒΡ≈®Ε»ΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓmol/LΘΜΧλ»ΜΦνBΒΡΜ·―ß ΫΈΣΘΚΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ ΓΓΓΓΓΘ
Θ®3Θ©»τ»Γ“ΜΕ®÷ ΝΩΒΡΧλ»ΜΦνBΦ”»κΒΫ30mLΗΟ≈®Ε»ΒΡ―ΈΥα÷–Θ§«κ–¥≥ω≤ζ…ζΤχΧεΧεΜΐVΘ®mLΘ§±ξΉΦΉ¥ΩωΘ©”κΧλ»ΜΦνB÷ ΝΩW(g) ÷°ΦδΒΡΙΊœΒ ΫΓΘ
‘ΎΜ®ΤΩ÷–Φ”»κΓΑœ Μ®±Θœ ΦΝΓ±Θ§Ρή―”≥Λœ Μ®ΒΡ ΌΟϋΓΘ
Θ®1Θ©œ÷”–“Μ÷÷Έό…ΪΒΡœ Μ®”Σ―χ“ΚΘ§Ω…Ρή”…œθΥαΗΤΓΔΧΦΥαΦΊΓΔœθΥαΦΊΓΔ¬»Μ·ΦΊ÷–ΒΡ“Μ÷÷ΜρΦΗ÷÷Έο÷ Ήι≥…Θ§ΈΣΧΫΨΩΤδ≥…Ζ÷Θ§Ρ≥Ά§―ß…ηΦΤ≤ΔΆξ≥…ΝΥ»γœ¬ΆΦΥυ ΨΒΡ Β―ιΓΘ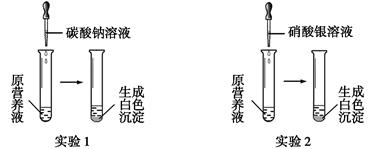
ΗυΨί“‘…œ Β―ιΘ§«κΡψΧνΩ’ΓΘ
ΔΌ”… Β―ι1Ω…»ΖΕ®‘≠”Σ―χ“Κ÷–“ΜΕ®ΟΜ”–ΒΡΈο÷ « (ΧνΜ·―ß Ϋ)Θ§–¥≥ω…ζ≥…ΑΉ…Ϊ≥ΝΒμΒΡάκΉ”ΖΫ≥Χ Ϋ « ΓΘ
ΔΎ»τ≤βΒΟ‘≠”Σ―χ“Κ÷–K+ΓΔCl-ΒΡ ΐΡΩ÷°±»ΈΣ2ΓΟ1Θ§‘ρ‘≠”Σ―χ“Κ «”… ÷÷»ή÷ ≈δ÷Τ≥…ΒΡΓΘ
ΔέΡ≥Ά§―ß”Ο¬»Μ·ΗΤΓΔœθΥαΦΊΓΔ¬»Μ·ΦΊ≈δ≥…ΒΡ”Σ―χ“Κ÷–K+ΓΔCl-ΓΔNO3-ΒΡ ΐΡΩ÷°±»ΈΣ2ΓΟ5ΓΟ1Θ§
‘ρΥυ”ΟœθΥαΦΊΚΆ¬»Μ·ΗΤΒΡΈο÷ ΒΡΝΩ÷°±» « ΓΘ
Θ®2Θ©œ¬±μ «500mLΡ≥ΓΑœ Μ®±Θœ ΦΝΓ±÷–Κ§”–ΒΡ≥…Ζ÷Θ§‘ΡΕΝΚσΜΊ¥πœ¬Ν–Έ ΧβΓΘ
| ≥…Ζ÷ | ÷ ΝΩΘ®gΘ© | ΡΠΕϊ÷ ΝΩΘ®g ΓΛmol-1Θ© |
| ’αΧ« | 68.4 | 342 |
| ΝρΥαΦΊ | 0.50 | 174 |
| ΑΔΥΨΤΞΝ÷ | 0.35 | 180 |
| ΗΏΟΧΥαΦΊ | 0.50 | 158 |
| œθΥα“χ | 0.04 | 170 |
ΔΌΓΑœ Μ®±Θœ ΦΝΓ±÷–’αΧ«ΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ___________________ΓΘ
ΔΎ≈δ÷ΤΗΟ500mL ΓΑœ Μ®±Θœ ΦΝΓ±Υυ–ηΒΡ≤ΘΝß“«Τς≥ΐΝΥ…’±≠ΓΔ≤ΘΝßΑτΓΔΝΩΆ≤ΆβΜΙ”– ΓΘ
Δέ‘Ύ»ή“Κ≈δ÷ΤΙΐ≥Χ÷–Θ§œ¬Ν–≤ΌΉςΡή Ι≈δ÷ΤΫαΙϊΤΪ–ΓΒΡ «___________ΓΘ
AΘ°Ε®»ί ±―ω ”»ίΝΩΤΩΩΧΕ»œΏ
BΘ°»ίΝΩΤΩ‘Ύ Ι”Ο«ΑΈ¥Η…‘οΘ§άοΟφ”–…ΌΝΩ’τΝσΥ°
CΘ°“Τ“Κ ±Θ§“ΚΧε≤Μ–Γ–Ρ¥”Άβ±ΎΝς≥ω
DΘ°Ε®»ί“Γ‘»ΚσΖΔœ÷“ΚΟφΒΆ”Ύ»ίΝΩΤΩΒΡΩΧΕ»œΏΘ§ΒΪΈ¥Ήω»ΈΚΈ¥Πάμ