��Ŀ����
I����ú��Ϊȼ�Ͽ�ͨ����������;����
;����C��s��+O2��g���TCO2��g������H1��0��
;�������Ƴ�ˮú����
C��s��+H2O��g���TCO��g��+H2��g������H2��0��
��ȼ��ˮú����
2CO��g��+O2��g���T2CO2��g������H3��0��
2H2��g��+O2��g���T2H2O��g������H4��0��
��ش��������⣺
��1��;����ų�������������______���������=��������;����ų���������
��2����H1����H2����H3����H4����ѧ��ϵʽ��______��
��3����֪����C��s��+O2��g��=CO2��g������H=-393.5kJ?mol-1
��2CO��g��+O2��g��=2CO2��g������H=-566kJ?mol-1
��TiO2��s��+2Cl2��g��=TiCl4��s��+O2��g������H=+141kJ?mol-1
��TiO2��s��+2Cl2��g��+2C��s��=TiCl4��s��+2CO��g���ġ�H=______��
��1����25�桢101kPa�£�1gҺ̬�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����
227kJ����÷�Ӧ���Ȼ�ѧ����ʽӦΪ______��
��2����������������Ӧ����1molҺ̬ˮʱ����285.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ______����1gˮ����ת����Һ̬ˮ����2.444kJ����Ӧ2H2��g��+O2��g���T2H2O��g���ġ�H=______��
;����C��s��+O2��g���TCO2��g������H1��0��
;�������Ƴ�ˮú����
C��s��+H2O��g���TCO��g��+H2��g������H2��0��
��ȼ��ˮú����
2CO��g��+O2��g���T2CO2��g������H3��0��
2H2��g��+O2��g���T2H2O��g������H4��0��
��ش��������⣺
��1��;����ų�������������______���������=��������;����ų���������
��2����H1����H2����H3����H4����ѧ��ϵʽ��______��
��3����֪����C��s��+O2��g��=CO2��g������H=-393.5kJ?mol-1
��2CO��g��+O2��g��=2CO2��g������H=-566kJ?mol-1
��TiO2��s��+2Cl2��g��=TiCl4��s��+O2��g������H=+141kJ?mol-1
��TiO2��s��+2Cl2��g��+2C��s��=TiCl4��s��+2CO��g���ġ�H=______��
��1����25�桢101kPa�£�1gҺ̬�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����
227kJ����÷�Ӧ���Ȼ�ѧ����ʽӦΪ______��
��2����������������Ӧ����1molҺ̬ˮʱ����285.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ______����1gˮ����ת����Һ̬ˮ����2.444kJ����Ӧ2H2��g��+O2��g���T2H2O��g���ġ�H=______��
��1�����ݸ�˹���ɣ���Ӧ��ֻ��ʼ̬����̬�йأ���;���أ�;������;�����ʼ̬��ͬ����̬��ͬ��Ӧ����ȣ�
�ʴ�Ϊ����ȣ�
��2��C��s��+H2O��g���TCO��g��+H2��g������H2��0��
2CO��g��+O2��g���T2CO2��g������H3��0��
2H2��g��+O2��g���T2H2O��g������H4��0��
���ݸ�˹���ɣ���+�ۡ�
+�ܡ�
��C��s��+O2��g���TCO2��g�����ʡ�H1=��H2+
����H3+��H4��
�ʴ�Ϊ����H1=��H2+
����H3+��H4����
��3����֪����C��s��+O2��g��=CO2��g������H=-393.5kJ?mol-1
��2CO��g��+O2��g��=2CO2��g������H=-566kJ?mol-1
��TiO2��s��+2Cl2��g��=TiCl4��s��+O2��g������H=+141kJ?mol-1
���ݸ�˹���ɣ��١�2-��+�۵�TiO2��s��+2Cl2��g��+2C��s��=TiCl4��s��+2CO��g�����ʡ�H=2����-393.5kJ?mol-1-��-566kJ?mol-1��+141kJ?mol-1=-80kJ?mol-1��
�ʴ�Ϊ��-80kJ?mol-1��
��1��1mol�״�������Ϊ1mol��32g/mol=32g��1gҺ̬�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.7kJ����32g�״�ȼ������CO2��Һ̬ˮʱ����22.7kJ��
=726.4���ʷ�Ӧ���Ȼ�ѧ����ʽΪ��CH3OH��l��+
O2��g���TCO2��g��+2H2O��l����H=-726.4kJ?mol-1��
�ʴ�Ϊ��CH3OH��l��+
O2��g���TCO2��g��+2H2O��l����H=-726.4kJ?mol-1��
��2��������������Ӧ����1molҺ̬ˮʱ����285.8kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��H2��g��+
O2��g���TH2O��l����H=-285.8kJ?mol-1��
1gˮ����ת����Һ̬ˮ����2.444kJ��2molˮ������Ϊ2mol��18g/mol=36g����2molҺ̬ˮת��Ϊ2mol��̬ˮ���յ�����Ϊ2.444kJ��
=88kJ����������������Ӧ����2mol��̬ˮ�ķ�Ӧ��Ϊ��H=-��285.8��2-88��kJ/mol=-483.6kJ/mol��
�ʴ�Ϊ��-483.6kJ/mol��
�ʴ�Ϊ����ȣ�
��2��C��s��+H2O��g���TCO��g��+H2��g������H2��0��
2CO��g��+O2��g���T2CO2��g������H3��0��
2H2��g��+O2��g���T2H2O��g������H4��0��
���ݸ�˹���ɣ���+�ۡ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ����H1=��H2+
| 1 |
| 2 |
��3����֪����C��s��+O2��g��=CO2��g������H=-393.5kJ?mol-1
��2CO��g��+O2��g��=2CO2��g������H=-566kJ?mol-1
��TiO2��s��+2Cl2��g��=TiCl4��s��+O2��g������H=+141kJ?mol-1
���ݸ�˹���ɣ��١�2-��+�۵�TiO2��s��+2Cl2��g��+2C��s��=TiCl4��s��+2CO��g�����ʡ�H=2����-393.5kJ?mol-1-��-566kJ?mol-1��+141kJ?mol-1=-80kJ?mol-1��
�ʴ�Ϊ��-80kJ?mol-1��
��1��1mol�״�������Ϊ1mol��32g/mol=32g��1gҺ̬�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.7kJ����32g�״�ȼ������CO2��Һ̬ˮʱ����22.7kJ��
| 32g |
| 1g |
| 3 |
| 2 |
�ʴ�Ϊ��CH3OH��l��+
| 3 |
| 2 |
��2��������������Ӧ����1molҺ̬ˮʱ����285.8kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��H2��g��+
| 1 |
| 2 |
1gˮ����ת����Һ̬ˮ����2.444kJ��2molˮ������Ϊ2mol��18g/mol=36g����2molҺ̬ˮת��Ϊ2mol��̬ˮ���յ�����Ϊ2.444kJ��
| 36g |
| 1g |
�ʴ�Ϊ��-483.6kJ/mol��

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ
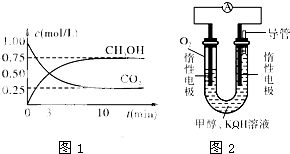
 a��c(Na+)= c(H2S)+c(HS?)+2c(S2?)
a��c(Na+)= c(H2S)+c(HS?)+2c(S2?) ��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��
��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��