��Ŀ����
������ʹ�������Դ��չ����̼���á�������Ϊ��ѧ���о�����Ҫ���⣮�������״������ʵ����ȼ�ϣ�������ȼ�ϵ�أ�
��1����֪����2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H1=-1275.6kJ?mol-1
��2CO��g��+O2��g��=2CO2��g����H2=-566.0kJ?mol-1
��H2O��g��=H2O��l����H3=-44.0kJ?mol-1д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��______��
��2�������״���ԭ��CO��H2��Դ�ڣ�CH4��g��+H2O��g��?CO��g��+3H2��g��
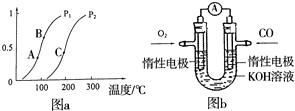
��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����Pl______P2��A��B��C���㴦��Ӧƽ�ⳣ����KA��KB��KC���Ĵ�С˳��Ϊ______�������������������=����
��100��ʱ����1molCH4��2molH2Oͨ���ݻ�Ϊ100L�ķ�Ӧ�ң���Ӧ��ƽ��ı�־�ǣ�______��
a�������������ܶȺ㶨
b����λʱ��������0.1molCH4ͬʱ����0.3molH2
c��������ѹǿ�㶨
d��3v����CH4��=v����H2��
����ﵽƽ��ʱCH4��ת����Ϊ0.5����100��ʱ�÷�Ӧ��ƽ�ⳣ��K=______
��3��ijʵ��С������CO��g����O2��g����KOH��aq����Ƴ���ͼb��ʾ�ĵ��װ�ã������ĵ缫��ӦʽΪ______���ø�ԭ�������Դ�������£��ö��Ե缫���200mL����ʳ��ˮ�������������ĵı�״���µ�CO224mL������Һ��pH=______������������Һ����ı仯��
��4������ȼ�ϵ�ص������ŵ��ǣ�______��______��������������
��1����֪����2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H1=-1275.6kJ?mol-1
��2CO��g��+O2��g��=2CO2��g����H2=-566.0kJ?mol-1
��H2O��g��=H2O��l����H3=-44.0kJ?mol-1д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��______��
��2�������״���ԭ��CO��H2��Դ�ڣ�CH4��g��+H2O��g��?CO��g��+3H2��g��
��һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����Pl______P2��A��B��C���㴦��Ӧƽ�ⳣ����KA��KB��KC���Ĵ�С˳��Ϊ______�������������������=����
��100��ʱ����1molCH4��2molH2Oͨ���ݻ�Ϊ100L�ķ�Ӧ�ң���Ӧ��ƽ��ı�־�ǣ�______��
a�������������ܶȺ㶨
b����λʱ��������0.1molCH4ͬʱ����0.3molH2
c��������ѹǿ�㶨
d��3v����CH4��=v����H2��
����ﵽƽ��ʱCH4��ת����Ϊ0.5����100��ʱ�÷�Ӧ��ƽ�ⳣ��K=______
��3��ijʵ��С������CO��g����O2��g����KOH��aq����Ƴ���ͼb��ʾ�ĵ��װ�ã������ĵ缫��ӦʽΪ______���ø�ԭ�������Դ�������£��ö��Ե缫���200mL����ʳ��ˮ�������������ĵı�״���µ�CO224mL������Һ��pH=______������������Һ����ı仯��
��4������ȼ�ϵ�ص������ŵ��ǣ�______��______��������������

��1����֪����2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��g����H=-1275.6kJ/mol
��2CO��g��+O2��g���T2CO2��g����H=-566.0kJ/mol
��H2O��g���TH2O��l����H=-44.0kJ/mol
�ɸ�˹���ɣ���-��+�ۡ�4�ã�
2CH3OH��l��+2O2��g���T2CO��g��+4H2O��l�����ʡ�H=-1275.6kJ�Mmol-��-566.0kJ/mol��+��-44.0kJ/mol����4=-885.6kJ�Mmol��
�������Ȼ�ѧ��Ӧ����ʽΪ��CH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-442.8kJ�Mmol��
�ʴ�Ϊ��CH3OH��1��+O2��g��=CO��g��+2H2O��1����H=-442.8kJ?mol-1��
��2���ٸ���һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵͼ֪�����¶Ȳ��䣬������ѹǿ��ƽ��CH4��g��+H2O��g��?CO��g��+3H2��g�������ƶ��������ת���ʼ�С������Pl��P2��ѹǿ���������¶ȣ������ת���ʻ�����ƽ��������У����Է�Ӧ�����ȷ�Ӧ���¶�Խ�ߣ�KԽ��A��B��C���㴦��Ӧ���¶��������ߵģ�����ƽ�ⳣ����KA��KB��KC���Ĵ�С˳��ΪKC��KB��KA��
�ʴ�Ϊ������KC��KB��KA��
��a�������������ܶ�=
���������غ�ģ�V�Dz���ģ������ܶ�ʼ�ղ��䣬���ܶȲ���ʱ����һ��ƽ�⣬�ʴ���
b����λʱ��������0.1molCH4ͬʱ����0.3molH2��ֻ��˵������Ӧ���ʣ�����֤�����淴Ӧ������ȣ���һ��ƽ�⣬�ʴ���
c����Ӧ��ǰ��ϵ���ͱ仯�ķ�Ӧ����������ѹǿ�㶨����ﵽ��ƽ�⣬����ȷ��
d��3v����CH4��=v����H2����������v������=v���棩������ȷ��
��ѡcd��
CH4��g��+H2O��g��?CO��g��+3H2��g��
��ʼŨ�ȣ�0.01 0.0200
�仯Ũ�ȣ�0.005 0.0050.0050.015
ƽ��Ũ�ȣ�0.0050.015 0.0050.015
����ƽ�ⳣ��K=
=2.25��10-4��mol/L��2��
�ʴ�Ϊ��cd��2.25��10-4��mol/L��2��
��3����CO��g����O2��g����KOH��aq����Ƴɵ�ȼ�ϵ��װ���У�CO����������Ӧ���ڸ���ʧȥ���ӣ���������������̼�����ˮ�������缫��ӦʽΪ��CO-2e-+4OH-=CO32-+2H2O���ø�ԭ�������Դ�������£��ö��Ե缫���200mL����ʳ��ˮ���������������ĵı�״���µ�CO224mL��0.01molʱ��ת�Ƶ�����0.02mol�����ݵ���Ȼ��Ƶķ�Ӧ����ʽ��2NaCl+2H2O
2NaOH+Cl2��+H2������ת�Ƶ�����0.02molʱ�������������Ƶ����ʵ�����0.02mol��������������Ũ����
=0.1mol/L����pH=13���ʴ�Ϊ��CO-2e-+4OH-=CO32-+2H2O��13��
��4��ԭ��ؾ������������ʸߵ��ص㣬����ȼ�ϵ�صIJ�����ˮ������Ⱦ���������ʴ�Ϊ�����������ʸߣ�����Ⱦ��������
��2CO��g��+O2��g���T2CO2��g����H=-566.0kJ/mol
��H2O��g���TH2O��l����H=-44.0kJ/mol
�ɸ�˹���ɣ���-��+�ۡ�4�ã�
2CH3OH��l��+2O2��g���T2CO��g��+4H2O��l�����ʡ�H=-1275.6kJ�Mmol-��-566.0kJ/mol��+��-44.0kJ/mol����4=-885.6kJ�Mmol��
�������Ȼ�ѧ��Ӧ����ʽΪ��CH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-442.8kJ�Mmol��
�ʴ�Ϊ��CH3OH��1��+O2��g��=CO��g��+2H2O��1����H=-442.8kJ?mol-1��
��2���ٸ���һ��������CH4��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵͼ֪�����¶Ȳ��䣬������ѹǿ��ƽ��CH4��g��+H2O��g��?CO��g��+3H2��g�������ƶ��������ת���ʼ�С������Pl��P2��ѹǿ���������¶ȣ������ת���ʻ�����ƽ��������У����Է�Ӧ�����ȷ�Ӧ���¶�Խ�ߣ�KԽ��A��B��C���㴦��Ӧ���¶��������ߵģ�����ƽ�ⳣ����KA��KB��KC���Ĵ�С˳��ΪKC��KB��KA��
�ʴ�Ϊ������KC��KB��KA��
��a�������������ܶ�=
| m |
| V |
b����λʱ��������0.1molCH4ͬʱ����0.3molH2��ֻ��˵������Ӧ���ʣ�����֤�����淴Ӧ������ȣ���һ��ƽ�⣬�ʴ���
c����Ӧ��ǰ��ϵ���ͱ仯�ķ�Ӧ����������ѹǿ�㶨����ﵽ��ƽ�⣬����ȷ��
d��3v����CH4��=v����H2����������v������=v���棩������ȷ��
��ѡcd��
CH4��g��+H2O��g��?CO��g��+3H2��g��
��ʼŨ�ȣ�0.01 0.0200
�仯Ũ�ȣ�0.005 0.0050.0050.015
ƽ��Ũ�ȣ�0.0050.015 0.0050.015
����ƽ�ⳣ��K=
| 0.0153��0.005 |
| 0.005��0.015 |
�ʴ�Ϊ��cd��2.25��10-4��mol/L��2��
��3����CO��g����O2��g����KOH��aq����Ƴɵ�ȼ�ϵ��װ���У�CO����������Ӧ���ڸ���ʧȥ���ӣ���������������̼�����ˮ�������缫��ӦʽΪ��CO-2e-+4OH-=CO32-+2H2O���ø�ԭ�������Դ�������£��ö��Ե缫���200mL����ʳ��ˮ���������������ĵı�״���µ�CO224mL��0.01molʱ��ת�Ƶ�����0.02mol�����ݵ���Ȼ��Ƶķ�Ӧ����ʽ��2NaCl+2H2O
| ||
| 0.02mol |
| 0.2L |
��4��ԭ��ؾ������������ʸߵ��ص㣬����ȼ�ϵ�صIJ�����ˮ������Ⱦ���������ʴ�Ϊ�����������ʸߣ�����Ⱦ��������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
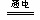 2 H2����O2������������ˮ�ķֽⷴӦ���Է���Ӧ
2 H2����O2������������ˮ�ķֽⷴӦ���Է���Ӧ O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1
O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1 O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1
O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1 O2��g���TCO��g����H1����110��5kJ/mol
O2��g���TCO��g����H1����110��5kJ/mol