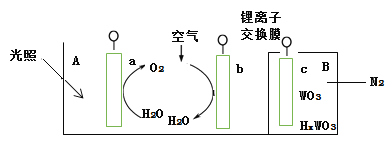
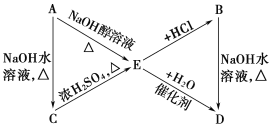
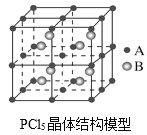
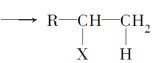Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņ Ķ—ť “÷–”–“Ľőī÷™Ň®∂»ĶńŌ°—őňŠ£¨ń≥Õ¨—ßő™≤‚∂®ł√—őňŠĶńŇ®∂»£¨‘ŕ Ķ—ť “÷–ĹÝ––Ńň»ÁŌ¬ Ķ—ť°£
£®1£©Ňš÷∆100 mL 0.10 mol/L NaOHĪÍ◊ľ»‹“ļ°£
ĘŔ÷ų“™≤Ŕ◊ų≤Ĺ÷Ť£ļľ∆ň„°ķ≥∆ŃŅ°ķ»‹Ĺ‚°ķņš»ī°ķ◊™“∆°ķŌīĶ”(≤ĘĹęŌīĶ”“ļ“∆»Ž»›ŃŅ∆Ņ)°ķ’ŮĶī°ķ_________°ķ__________°ķ◊į∆Ņ°ķŐýĪÍ«©°£
Ęŕ≥∆ŃŅ________g«‚—űĽĮń∆ĻŐŐŚ∑Řń©£¨ňý–Ť“«∆ų”–£ļÕ–ŇŐŐž∆Ĺ(īÝŪņ¬ŽļÕńų◊”)°Ę___________°Ę–°…’∆Ņ°£
£®2£©»°20.00 mLīż≤‚—őňŠ»‹“ļ∑Ň»Ž◊∂–ő∆Ņ÷–£¨≤ĘĶőľ”2°ę3Ķő∑”Ő™◊ų÷ł ĺľŃ£¨”√◊‘ľļŇš÷∆ĶńĪÍ◊ľ“ļNaOH»‹“ļĹÝ––Ķő∂®°£÷ōłī…Ō ŲĶő∂®≤Ŕ◊ų2°ę3īő£¨ľ«¬ľ żĺ›»ÁŌ¬£ļ
Ķ—ť ĪŗļŇ | NaOH»‹“ļĶńŇ®∂»(mol/L) | Ķő∂®ÕÍ≥… Ī£¨NaOH»‹“ļĶő»ŽĶńŐŚĽż(mL) | īż≤‚—őňŠ»‹“ļĶńŐŚĽż(mL) | |
1 | 0.10 | 29.80 | 20.00 | |
2 | 0.10 | 30.00 | 20.00 | |
3 | 0.10 | 30.20 | 20.00 |
ĘŔĶő∂®īÔĶĹ÷’Ķ„ĶńĪÍ÷ĺ «___________________________°£
Ęŕłýĺ›…Ō Ų żĺ›£¨Ņ…ľ∆ň„≥Ųł√—őňŠĶńŇ®∂»‘ľő™_______(Ī£ŃŰŃĹőĽ”––ß ż◊÷)°£
ĘŘŇŇ»•ľÓ ĹĶő∂®Ļ‹÷–∆ÝŇ›Ķń∑Ĺ∑®”¶≤…”√»ÁÕľňý ĺ≤Ŕ◊ų÷–Ķń____________£®—°‘Ůľ◊°Ę““°ĘĪŻŐÓŅ’£©£¨»Ľļů«Š«Šľ∑—Ļ≤£Ńß«Ú Ļľ‚◊ž≤Ņ∑÷≥š¬ķľÓ“ļ°£
Ę‹‘ŕ…Ō Ų Ķ—ť÷–£¨Ō¬Ń–≤Ŕ◊ų(∆šňŻ≤Ŕ◊ų’ż»∑)ĽŠ‘ž≥…≤‚∂®ĹŠĻŻ∆ęłŖĶń”–_______°£
A£ģĶő∂®÷’Ķ„∂Ń ż Īł© ”∂Ń ż
B£ģňŠ ĹĶő∂®Ļ‹ Ļ”√«į£¨ňģŌīļůőī”√īż≤‚—őňŠ»‹“ļ»ůŌī
C£ģ◊∂–ő∆ŅňģŌīļůőīł…‘Ô
D£ģĶő∂®Ļż≥Ő÷–£¨”–…ŔŃŅĪÍ◊ľ“ļŶ≥Ų◊∂–ő∆ŅÕ‚
E£ģľÓ ĹĶő∂®Ļ‹ľ‚◊ž≤Ņ∑÷”–∆ÝŇ›£¨Ķő∂®ļůŌŻ ß
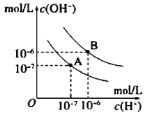
°ĺīūįł°Ņ∂®»› “°‘» 0.4 “©≥◊ ◊Óļůľ”»Ž“ĽĶőNaOH»‹“ļ£¨»‹“ļ”…őř…ę«°ļ√Īš≥…«≥ļž…ę«“įŽ∑÷÷”ńŕ«≥ļž…ę≤ĽÕ »• 0.15mol/L ĪŻ DE
°ĺĹ‚őŲ°Ņ
£®1£©»‹“ļŇš÷∆÷ų“™≤Ŕ◊ų≤Ĺ÷Ť£ļľ∆ň„°ķ≥∆ŃŅ°ķ»‹Ĺ‚°ķņš»ī°ķ◊™“∆°ķŌīĶ”(≤ĘĹęŌīĶ”“ļ“∆»Ž»›ŃŅ∆Ņ)°ķ’ŮĶī°ķ∂®»›°ķ“°‘»°ķ◊į∆Ņ°ķŐýĪÍ«©£Ľłýĺ›![]() ņīľ∆ň„£Ľłýĺ›≥∆ŃŅ«‚—űĽĮń∆ĻŐŐŚňý–Ť“«∆ųĹÝ––Ĺ‚īū£Ľ
ņīľ∆ň„£Ľłýĺ›≥∆ŃŅ«‚—űĽĮń∆ĻŐŐŚňý–Ť“«∆ųĹÝ––Ĺ‚īū£Ľ
£®2£©ĘŔ»Á»‹“ļ—’…ęĪšĽĮ«“įŽ∑÷÷”ńŕ≤ĽĪš…ę£¨Ņ…ňĶ√ųīÔĶĹĶő∂®÷’Ķ„£Ľ
Ęŕłýĺ›Ķő∂®ŌŻļńĶńĪÍ◊ľ“ļĶńŐŚĽżŇ–∂Ō żĺ›Ķń”––ß–‘£¨»Ľļůľ∆ň„≥ŲĪÍ◊ľ“ļĶń∆ĹĺýŐŚĽż£¨◊Óļůłýĺ›ĪÍ![]() ľ∆ň„≥Ųł√—őňŠĶńŇ®∂»£Ľ
ľ∆ň„≥Ųł√—őňŠĶńŇ®∂»£Ľ
ĘŘľÓ ĹĶő∂®Ļ‹Ķń∆ÝŇ›Õ®≥£Ōū∆§Ļ‹ńŕ£¨÷Ľ“™ĹęĶő∂®≤£ŃßÕ∑≥Į…Ō£¨≤Ęľ∑Ōū∆§Ļ‹÷–Ķń≤£Ńß÷ťĺÕŅ…“‘Ĺę∆ÝŇ›≥ŚŇŇ≥Ų£Ľ
Ę‹łýĺ›![]() ∑÷őŲ≤ĽĶĪ≤Ŕ◊ų∂‘V£®ĪÍ◊ľ£©Ķń”įŌž£¨“‘īňŇ–∂ŌŇ®∂»Ķńőů≤Ó°£
∑÷őŲ≤ĽĶĪ≤Ŕ◊ų∂‘V£®ĪÍ◊ľ£©Ķń”įŌž£¨“‘īňŇ–∂ŌŇ®∂»Ķńőů≤Ó°£
£®1£©ĘŔŇš÷∆100mL 0.10mol/L NaOHĪÍ◊ľ»‹“ļĶń≤Ĺ÷Ťő™£ļľ∆ň„°ķ≥∆ŃŅ°ķ»‹Ĺ‚°ķ£®ņš»īļů£©◊™“∆°ķŌīĶ”£®≤ĘĹęŌīĶ”“ļ“∆»Ž»›ŃŅ∆Ņ£©°ķ∂®»›°ķ“°‘»°ķĹęŇš÷∆ļ√Ķń»‹“ļĶĻ»Ž ‘ľŃ∆Ņ÷–£¨Őý…ŌĪÍ«©£¨
Ļ īūįłő™£ļ∂®»›£Ľ“°‘»£Ľ
Ęŕ–Ť“™Ķń«‚—űĽĮń∆Ķń÷ ŃŅm=nM=cVM=0.1L°Ń0.10molL-1°Ń40g/mol=0.4g£Ľ≥∆ŃŅ0.4g«‚—űĽĮń∆ĻŐŐŚňý–Ť“«∆ų£¨–Ť“™≥∆ŃŅ“«∆ųŐž∆Ĺ£®īÝŪņ¬Ž°Ęńų◊”£©£¨«‚—űĽĮń∆ĺŖ”–łĮ ī–‘£¨”¶ł√∑Ň‘ŕ…’Ī≠÷–≥∆ŃŅ£¨ĽĻ–Ť“™»°«‚—űĽĮń∆Ķń“«∆ų“©≥◊£Ľ
Ļ īūįłő™£ļ0.4£Ľ“©≥◊£Ľ
£®2£©ĘŔĶő∂®ĹŠ Ý«į—őňŠ÷–Ķő»Ž∑”Ő™£¨»‹“ļő™őř…ę£¨Ķő∂®ĹŠ Ý Ī«‚—űĽĮń∆ĻżŃŅ£¨»‹“ļĪš≥…ļž…ę£¨ňý“‘Ķő∂®÷’Ķ„Ō÷Ōůő™£ļ◊Óļů“ĽĶő«‚—űĽĮń∆»‹“ļľ”»Ž£¨»‹“ļ”…őř…ę«°ļ√Īš≥…«≥ļž…ę£¨«“įŽ∑÷÷”ńŕ«≥ļž…ę≤ĽÕ »•£Ľ
Ļ īūįłő™£ļ◊Óļůľ”»Ž“ĽĶőNaOH»‹“ļ£¨»‹“ļ”…őř…ę«°ļ√Īš≥…«≥ļž…ę«“įŽ∑÷÷”ńŕ«≥ļž…ę≤ĽÕ »•£Ľ
Ę໿īőĶő∂® żĺ›∂ľ «”––ßĶń£¨ŌŻļńĪÍ◊ľ“ļĶń∆ĹĺýŐŚĽżő™£ļ![]() £¨ł√—őňŠĶńŇ®∂»ő™£ļ
£¨ł√—őňŠĶńŇ®∂»ő™£ļ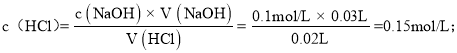
Ļ īūįłő™£ļ0.15mol/L£Ľ
ĘŘľÓ ĹĶő∂®Ļ‹Ķń∆ÝŇ›Õ®≥£Ōū∆§Ļ‹ńŕ£¨÷Ľ“™ĹęĶő∂®≤£ŃßÕ∑≥Į…Ō£¨≤Ęľ∑Ōū∆§Ļ‹÷–Ķń≤£Ńß÷ťĺÕŅ…“‘Ĺę∆ÝŇ›≥ŚŇŇ≥Ų£Ľ
Ļ īūįłő™£ļĪŻ£Ľ
Ę‹A£ģĶő∂®÷’Ķ„∂Ń ż Īł© ”∂Ń£¨‘ž≥…V£®ĪÍ◊ľ£©∆ę–°£¨łýĺ›![]() ∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ę–°£¨Ļ AīŪőů£Ľ
∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ę–°£¨Ļ AīŪőů£Ľ
B£ģňŠ ĹĶő∂®Ļ‹ Ļ”√«į£¨ňģŌīļůőī”√īż≤‚—őňŠ»ůŌī£¨īż≤‚“ļĪĽŌ° Õ£¨∆šőÔ÷ ĶńŃŅ∆ę–°£¨‘ž≥…V£®ĪÍ◊ľ£©∆ę–°£¨łýĺ›![]() ∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ę–°£¨Ļ BīŪőů£Ľ
∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ę–°£¨Ļ BīŪőů£Ľ
C£ģ◊∂–ő∆ŅňģŌīļůőīł…‘Ô£¨īż≤‚“ļĶńőÔ÷ ĶńŃŅ≤ĽĪš£¨∂‘V£®ĪÍ◊ľ£©őř”įŌž£¨łýĺ›![]() ∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©≤ĽĪš£¨Ļ CīŪőů£Ľ
∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©≤ĽĪš£¨Ļ CīŪőů£Ľ
D. Ķő∂®Ļż≥Ő÷–£¨”–…ŔŃŅĪÍ◊ľ“ļŶ≥Ų◊∂–ő∆ŅÕ‚Ķľ÷¬V£®ĪÍ◊ľ£©∆ęīů£¨łýĺ›![]() ∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ęīů£¨Ļ D’ż»∑£Ľ
∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ęīů£¨Ļ D’ż»∑£Ľ
E£ģĶő∂®«įĶő∂®Ļ‹ľ‚◊žī¶”–∆ÝŇ›£¨Ķő∂®ļů∆ÝŇ›ŌŻ ߣ¨‘ž≥…V£®ĪÍ◊ľ£©∆ęīů£¨łýĺ›![]() ∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ęīů£¨Ļ E’ż»∑£Ľ
∑÷őŲ£¨Ņ…÷™c£®īż≤‚£©∆ęīů£¨Ļ E’ż»∑£Ľ
Ļ —°DE°£

 ‘ń∂ŃŅž≥ĶŌĶŃ–īūįł
‘ń∂ŃŅž≥ĶŌĶŃ–īūįł