��Ŀ����
����Ŀ����֪��2H2O(l)��2H2(g)��O2(g) ��H����571.0 kJ/mol����̫����Ϊ��Դ�ֽ�Fe3O4�����Ȼ�ѧ����������ѭ���ֽ�ˮ��H2�Ĺ������£�
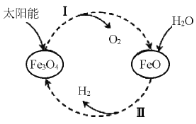
����I��2Fe3O4(s)��6FeO(s)��O2(g) ��H����313.2 kJ/mol
����II������
����˵������ȷ����
A.����I��ÿ����232gFe3O4ת��2mol���ӣ�
B.����II�Ȼ�ѧ����ʽΪ��3FeO(s)��H2O(l)��H2(g)��Fe3O4(s) ��H����128.9 kJ/mol
C.����I��II������ת������ʽ�����ǣ�̫���ܡ���ѧ�ܡ�����
D.����I������Ӧ�Ļ�ܴ����淴Ӧ�Ļ��
���𰸡�C
��������
A.���ݷ���ʽ2Fe3O4(s)��6FeO(s)��O2(g)������I��ÿ����232gFe3O4��1mol����������ת��2mol���ӣ���A��ȷ��
B.�� 2H2O(l)��2H2(g)��O2(g) ��H����571.0 kJ/mol����2Fe3O4(s)��6FeO(s)��O2(g) ��H����313.2 kJ/mol�����ݸ�˹���ɣ���![]() ����
����![]() �ù���II�Ȼ�ѧ����ʽΪ��3FeO(s)��H2O(l)��H2(g)��Fe3O4(s) ��H����128.9 kJ/mol����B��ȷ��
�ù���II�Ȼ�ѧ����ʽΪ��3FeO(s)��H2O(l)��H2(g)��Fe3O4(s) ��H����128.9 kJ/mol����B��ȷ��
C.����I��II������ת������ʽ�����ǣ�̫���ܡ���ѧ�ܣ���C����
D. 2Fe3O4(s)��6FeO(s)��O2(g) ��H����313.2 kJ/mol������Ӧ���ȣ����Թ���I������Ӧ�Ļ�ܴ����淴Ӧ�Ļ�ܣ���D��ȷ��
ѡC��

����Ŀ��ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijͬѧΪ�ⶨ�������Ũ�ȣ���ʵ�����н���������ʵ�顣
��1������100 mL 0.10 mol/L NaOH����Һ��
����Ҫ�������裺������������ܽ����ȴ��ת�ơ�ϴ��(����ϴ��Һ��������ƿ)����_________��__________��װƿ������ǩ��
�ڳ���________g�������ƹ����ĩ�����������У�������ƽ(�����������)��___________��С��ƿ��
��2��ȡ20.00 mL����������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�ҺNaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
ʵ�� ��� | NaOH��Һ��Ũ��(mol/L) | �ζ����ʱ��NaOH��Һ��������(mL) | ����������Һ�����(mL) | |
1 | 0.10 | 29.80 | 20.00 | |
2 | 0.10 | 30.00 | 20.00 | |
3 | 0.10 | 30.20 | 20.00 |
�ٵζ��ﵽ�յ�ı�־��___________________________��
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ_______(������λ��Ч����)��
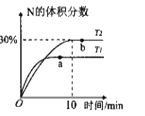
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е�____________��ѡ��ס��ҡ�����գ���Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
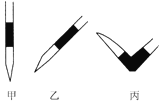
��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ���_______��
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D���ζ������У���������Һ������ƿ��
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
����Ŀ��ij��ѧ��ȤС��������������Һ��ͭ�۷�Ӧ������Ӧ����Һ�м���KSCN��Һ�Լ���Fe3+�Ƿ���ʣ�࣬ʵ���¼���£�
ʵ���� | ���� | ���� |
ʵ��1 |
| i������Cu�ۺ�������Һ������ ii��ȡ����i����Һ���Թ��У��μ�2�� 0.2mol/LKSCN��Һ����Һ��Ϊ��ɫ�������ɫѸ����ȥ���а�ɫ�������ɡ� |
��1��д��ʵ��1�е�i�������ӷ���ʽ_______________����ͬѧ�����ii�����ֵ��쳣������������Һ�е�Cu2+�����˼���Fe3+�������������������
��2Cu2++4SCN- ![]() 2CuSCN������ɫ��+(SCN)2����ɫ��
2CuSCN������ɫ��+(SCN)2����ɫ��
������[(SCN)2]����һ����±�أ�������±�ص������ƣ��������Խ���Br2��I2֮�䡣
��ͬѧ��ͨ������ʵ����֤����
ʵ���� | ���� | ���� |
ʵ��2 |
| ��Һ����ɫ��һ��ʱ����ʼ���ְ�ɫ�������ϲ���Һ��Ϊ��ɫ |
ʵ��3 |
| ��ɫ��Һ������죬ͬʱ���ɰ�ɫ������ |
��2������⣬ʵ��2��Ӧ�����ҺpHֵ��С�����ܵ�ԭ����___________________________________________��
��3������ʵ��2��3��ʵ������ͬѧ�ƶ�ʵ��3����Һ���������Fe2+��(SCN)2������д����Һ�������ӷ���ʽ_______________________��������ʵ��2�е���Һ��һ����������֤����һ���۵Ŀ����ԡ�

����ʵ��4��Ŀ�����ų�����Һ����Cu2+�Ŀ��ܣ���Ӧ��������____________________________________________��
��4����ͬѧͬʱ��Ϊ������������ԭ��Ӧԭ�����ڴ������£�Cu2+Ҳ������Fe2+�������ж�������_______��
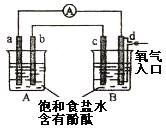
��5��Ϊ�ų����ţ�С��ͬѧ�����������װ�á�
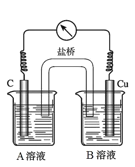
��A��ҺΪ____________________________��
����������ָ��ƫת��˵��Cu��Fe3+�����˷�Ӧ��������Ϊ����˵���Ƿ������__________________���������������ԭ����__________________________________________��
����֤Fe3+�Ƿ���뷴Ӧ�IJ�����________________________________________