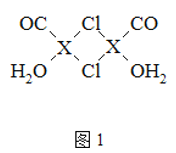��Ŀ����
����Ŀ����������68����ʱ���ҹ���һ�ҹ�����ĸ�ɹ���ˮ�����캽ĸ��Ҫ���������Ͳ��ϡ���ĸ������Ҫ�ͳ������ĸ�ļװ�Ҫ���£���ĸ�����Ҫ��ʴ��
(1)�����־��ǿ���ʴ����ǿ�����Ͳ��ϡ�
�ٻ�̬Niԭ�ӵĵ����Ų�ʽΪ________________����Ԫ�������ڱ���____����
��Ni����CO�γ����������ε������Ni(CO)4��1 mol Ni(CO)4�к���________ mol ������
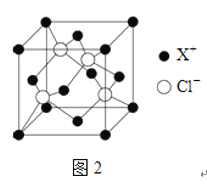
��NiO�ľ���ṹ��ͼ����ʾ����������������� A Ϊ(0,0,0)��BΪ(1,1,0)����C�����������Ϊ____��
(2)����ײ��д����ḻ�Ŀ�ȼ����һ�������£�CH4��CO2������H2O�γ���״�ṹ(����ͼ��ʾ)��ˮ���ᄃ�壬����ز������±���CH4��H2O�γɵ�ˮ���ᄃ���׳�����ȼ������
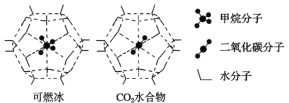
���� ���� | ����ֱ��/nm | ������H2O�Ľ���� E/kJ��mol��1 |
CH4 | 0.436 | 16.40 |
CO2 | 0.512 | 29.91 |
������ȼ�����з��Ӽ���ڵ�2����������_____________________��
��Ϊ�������������ȼ�������п�ѧ�������CO2�û�CH4�����롣��֪��ͼ����״�ṹ�Ŀ�ǻֱ��Ϊ0.586 nm����������ͼ���������ʽṹ�����ʵĽǶȷ������������������___________________________________��
(3)��CH4��CO2����������Ԫ�ص縺�Դ�С�����˳��Ϊ_______________
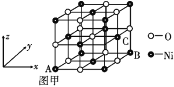
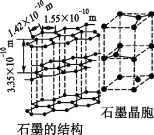
��̼����һ��ͬ������������ʯī���侧��ṹ��ͼ��ʾ����ʯī������̼ԭ�Ӹ���Ϊ____������֪ʯī���ܶ�Ϊ�� g��cm��3��C��C����Ϊr cm�������ӵ�������ֵΪNA������ʯī����IJ���Ϊ__________cm��
���𰸡�1s22s22p63s23p63d84s2��[Ar]3d84s2 d 8 ![]() ��������»��� CO2�ķ���ֱ��С����״�ṹ�Ŀ�ǻֱ��������H2O�Ľ��������CH4 H��C��O����H��C��O 4
��������»��� CO2�ķ���ֱ��С����״�ṹ�Ŀ�ǻֱ��������H2O�Ľ��������CH4 H��C��O����H��C��O 4 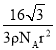
��������
(1)��̬��ԭ�ӵ�ԭ������Ϊ28�����ݺ�������Ų����ɺͺ��ع������𣻸���Ԫ�����ڱ��ṹ������Ni(CO)4��������������Niԭ�Ӻ�Cԭ�ӡ�CO������Cԭ�Ӻ�Oԭ��֮�䶼����������
(2)��ȼ���д���ˮ���ӣ�ˮ�����д��ڷ��Ӽ�����������������ݱ���ó�������̼�ķ���ֱ��С��0.586nm������ˮ�Ľ������Ϊ29.91����16.40��
(3)Ԫ�صķǽ�����Խǿ����縺��Խ��
(1) ��Ni��ԭ������Ϊ28��������Ų�ʽΪ[Ar]3d84s2��1s22s22p63s23p63d84s2����Ԫ���ڵ�������IVB�壬λ�������ڱ���d����
��Ni����CO�γ����������ε������Ni(CO)4�������д���4��Ni-C����Ϊ������C=O�ṹΪ��������1 mol Ni(CO)4�к���8mol ������
��NiO�ľ���ṹ��ͼ��ʾ����������������� A Ϊ(0,0,0)��BΪ(1,1,0)��Cλ�����ģ����ݾ�������ṹ��֪��C�����������Ϊ![]() ��
��
(2) CH4��H2O�γɵ�ˮ���ᄃ���׳�����ȼ������
�ٸ��ݽṹ�����Ӿ������������Ƿ��»�����ˮ����֮����������
���ɱ����֪��������̼�ķ���ֱ��С����״�ṹ�Ŀ�ǻֱ������0.512��0.586����˳��������״��ǻ�ڣ��Ҷ�����̼��ˮ�Ľ������ǿ�ڼ��飬�������ʽṹ�����ʵĽǶȷ������������������CO2�ķ���ֱ��С����״�ṹ�Ŀ�ǻֱ��������H2O�Ľ��������CH4��
(3)��Ԫ�صķǽ�����Խǿ����縺��Խ�����ڷǽ�����O��C��H���ʵ縺��H��C��O��
�ڸ��ݾ���ṹ��֪���辧���ĵױ߳�Ϊacm�������ĸ�Ϊhcm������Ϊdcm����h=2d������ͼΪ ����a/2��r��sin60�㣬�ɵ�a��
����a/2��r��sin60�㣬�ɵ�a��![]() ����������Ϊ
����������Ϊ![]() ��������Cԭ����ĿΪ
��������Cԭ����ĿΪ![]() ��4����������Ϊ
��4����������Ϊ![]() g����g��cm-3��
g����g��cm-3��![]() g��[
g��[![]() ��2d]cm3�������ɵ�d=
��2d]cm3�������ɵ�d=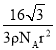 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����һ����������һ�ܱ������г���һ����CO2���壬�������������ۣ�������Ӧ��Fe(s)��CO2(g)![]() FeO(s)��CO(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
FeO(s)��CO(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
T/�� | 1200 | 1100 | 1000 | 900 | 800 |
K | 0.36 | 0.49 | 1.0 | 1.23 | 1.89 |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��______��
��2���÷�ӦΪ______(��������������������)��Ӧ��
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������____��
A��������ѹǿ���� B�����������c(CO)����
C����������ƽ����Է����������� D��c(CO2)��c(CO)
��4�����д�ʩ�У��ܹ��ı�ƽ��ʱc(CO)/c(CO2)�ı�ֵ����_____(�����)��
A���¶� B�����۵���(����)
C��ѹǿ D��CO����
��5����900���£��÷�Ӧ�ﵽƽ��ʱ��CO2�����ʵ���Ũ��Ϊ2mol��L-1�����ʱc(CO)��____ mol��L-1