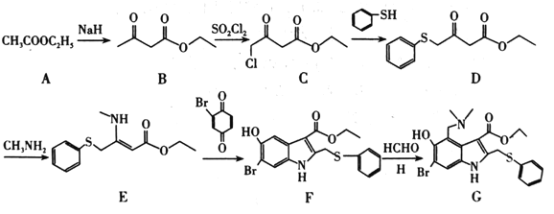
��Ŀ����
����Ŀ��ij����������ܺ���![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �е�һ�ֻ��֣����û�����������ʵ�飺
�е�һ�ֻ��֣����û�����������ʵ�飺
�ٽ�������������ˮ�еõ���ɫ��Һ�Ͱ�ɫ���������ˣ�
��ȡ��Һ������ɫ��Ӧ������ʻ�ɫ��
��ȡ��ɫ��������ϡ���ᣬ������ȫ�ܽⲢ�ų�����
�����������ƶϣ�
��1���û������һ������________��һ��������________�����ܺ���________��
��2����Ҫ������ܺ��е������Ƿ���ڣ����Բ��õ�ʵ�����Ϊ_____����д��ĸ����
A.ȡ���������Һ������![]() ��Һ
��Һ
B.ȡ���������Һ������NaOH��Һ
C.ȡ���������Һ����������![]() ��Һ�����ú����ϲ���Һ�м��������ữ
��Һ�����ú����ϲ���Һ�м��������ữ![]() ��Һ
��Һ
D.ȡ���������Һ����������![]() ��Һ�����ú��ϲ���Һ�м��������ữ
��Һ�����ú��ϲ���Һ�м��������ữ![]() ��Һ
��Һ
���𰸡�CaCO3��Na2SO4 Ba(NO3)2 ��CuSO4 MgCl2 BC
��������
���ݢٻ��������ˮ�õ���ɫ��Һ����CuSO4��Һ����ɫ�ģ�����ȷ��һ��������CuSO4�����ݢ���ɫ��Ӧ�ʻ�ɫ��˵������Na+��������п϶�����Na2SO4�����ݢ۰�ɫ��������ϡ���ᣬ������ȫ�ܽⲢ�ų����壬˵�������������Na2SO4��Ӧ���ɲ�����ϡ�����BaSO4������Ba(NO3)2һ�������ڣ���ԭ������һ������CaCO3�������������û������һ������CaCO3��Na2SO4��һ��������Ba(NO3)2 ��CuSO4�����ܺ���MgCl2���Դ˽��
��1���ɷ�����֪���û������һ������CaCO3��Na2SO4��һ��������Ba(NO3)2 ��CuSO4�����ܺ���MgCl2��
��2����Ҫ����MgCl2�Ƿ���ڣ�����ͨ���������е�Mg2+����Cl��������Mg2+�����ڲ��������Һ����NaOH��Һ�������Ƿ������ɫ������֤��������Cl�������ڲ��������Һ����AgNO3��Һ��������ԭ�����д���Na2SO4����AgNO3��Һ��ӦҲҪ���ɰ�ɫ�����������ȳ�ȥSO42������ȡ���������Һ����������Ba(NO3��2��Һ�����ú����ϲ���Һ�м����ữAgNO3��Һ����ѡBC��

����Ŀ����������68����ʱ���ҹ���һ�ҹ�����ĸ�ɹ���ˮ�����캽ĸ��Ҫ���������Ͳ��ϡ���ĸ������Ҫ�ͳ������ĸ�ļװ�Ҫ���£���ĸ�����Ҫ��ʴ��

(1)�����־��ǿ���ʴ����ǿ�����Ͳ��ϡ�
�ٻ�̬Niԭ�ӵĵ����Ų�ʽΪ________________����Ԫ�������ڱ���____����
��Ni����CO�γ����������ε������Ni(CO)4��1 mol Ni(CO)4�к���________ mol ������
��NiO�ľ���ṹ��ͼ����ʾ����������������� A Ϊ(0,0,0)��BΪ(1,1,0)����C�����������Ϊ____��
(2)����ײ��д����ḻ�Ŀ�ȼ����һ�������£�CH4��CO2������H2O�γ���״�ṹ(����ͼ��ʾ)��ˮ���ᄃ�壬����ز������±���CH4��H2O�γɵ�ˮ���ᄃ���׳�����ȼ������
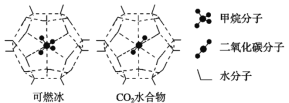
���� ���� | ����ֱ��/nm | ������H2O�Ľ���� E/kJ��mol��1 |
CH4 | 0.436 | 16.40 |
CO2 | 0.512 | 29.91 |
������ȼ�����з��Ӽ���ڵ�2����������_____________________��
��Ϊ�������������ȼ�������п�ѧ�������CO2�û�CH4�����롣��֪��ͼ����״�ṹ�Ŀ�ǻֱ��Ϊ0.586 nm����������ͼ���������ʽṹ�����ʵĽǶȷ������������������___________________________________��
(3)��CH4��CO2����������Ԫ�ص縺�Դ�С�����˳��Ϊ_______________
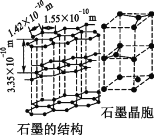
��̼����һ��ͬ������������ʯī���侧��ṹ��ͼ��ʾ����ʯī������̼ԭ�Ӹ���Ϊ____������֪ʯī���ܶ�Ϊ�� g��cm��3��C��C����Ϊr cm�������ӵ�������ֵΪNA������ʯī����IJ���Ϊ__________cm��
����Ŀ��ʵ���ҿ����û�������������Ӧ�Ʊ�����ͪ����Ӧԭ����ʵ��װ�ã����ּг�װ����ȥ�����£�

�й����ʵ��������ʼ��±���
���� | �е㣨�棩 | �ܶȣ�g/cm3��20�棩 | �ܽ��� |
������ | 161.1��97.8��* | 0.96 | ������ˮ���� |
����ͪ | 155.6��95.0��* | 0.95 | ����ˮ���������� |
ˮ | 100.0 | 1.0 |
*�����е����ݱ�ʾ���л�����ˮ�γɵľ��й̶���ɵĻ����ķе㡣
ʵ����ͨ��װ��B������Na2Cr2O7��Һ�ӵ�ʢ��10mL��������A�У���55��65����з�Ӧ����Ӧ��ɺ�������ˮ�������ռ�95��100�����֣��õ���Ҫ������ͪ��Ʒ��ˮ�Ļ���
(1)����D������Ϊ_____��
(2)�ٵμ�����Na2Cr2O7��Һʱ��Ӧ�����ӷ���ʽΪ______________��
�������ܷ��뻷��ͪ��ˮ��ԭ����_____��
(3)����ͪ��Ҫ�������²����ᴿ��
a.��Һ���м���NaCl���������ͣ����ã���Һ
b.ˮ�������ѣ����ѷе�34.6�棬��ȼ�գ���ȡ����ȡҺ�����л���
c.������ˮMgSO4���壬��ȥ�л�����������ˮ
d.����
e.����ȥ���Ѻ��ռ�151��156�����
��b��ˮ����������ȡ��Ŀ����_____��
����������a��d��ʹ�õIJ����������ձ����������⣬����Ҫ�IJ���������____��___������a�У�����NaCl�����������_____��
(4)�ָ�������ʱ������õ�����Ʒ���Ϊ8mL����ͪ�IJ���Ϊ____��������3λ��Ч���֣�