��Ŀ����
ij��ҵ��ˮ�������������е�5�֣�K+��Cu2+��Al3+��Fe2+��Cl����CO32����NO3����SO42�����Ҹ������ӵ����ʵ���Ũ����ȡ���ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ�ܲ����۲�����ɫ���档
����ȡ��Һ��������ϡ���ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䡣
����ȡ��Һ����BaCl2��Һ���а�ɫ�������ɡ�
��������ʵ�飬�����Ʋ���ȷ���ǣ�( )
| A����Һ������ȷ��Al3+�Ĵ������ |
| B��ԭ��Һ������������Ϊ��Cu2+��Fe2+��Cl����NO3����SO42�� |
| C��������п���ȷ��Fe2+��NO3���Ĵ��ڣ�����ȷ���������ӵĴ��� |
| D��������еİ�ɫ����Ϊ2�ֱ��� |
B
���������������ʵ��ٿ�֪��Һ����K+����ʵ��ڿ�֪��Fe2+��Cl����NO3������ʵ��ۿ�֪��Һ�к�SO42������Ϊ�����ӵ����ʵ���Ũ����ͬ�����Ի�Ӧ����һ��+2�۵������ӣ�ȷ����Cu2+���Ӷ�ȷ����ΪB��
���㣺���ӹ��桢�����ƶϡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��������ӷ���ʽ��ȷ���ǣ� ��
A��������̼������Һ��Ӧ��2H+��CO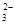 == CO2����H2O == CO2����H2O |
| B��������Һ������������ͭ��Ӧ��CH3COOH��OH�� ����CH3COO����H2O |
C����������Һ��ͨ������������̼��2C6H5O����CO2��H2O���� 2C6H5OH��CO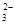 |
D����������Һ������ȩ�е�ȩ����CH3CHO +2[Ag(NH3)2]+ + 2OH�� CH3COO��+ NH4+ +3NH3 + 2Ag��+ H2O CH3COO��+ NH4+ +3NH3 + 2Ag��+ H2O |
���������������������ӷ���ʽ������ȷ���ǣ� ��
A������������Һ�е�����ɫʯ����Һ����ͨ��CO2����Һ������죺CO2��2OH����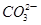 ��H2O ��H2O |
| B��Ư����Һ�м��Ȼ�����Һ�����������ɫ������Fe3����3ClO����3H2O��Fe��OH��3����3HClO |
| C��Cl2ͨ��FeBr2��Һ�У�Cl2��FeBr2���ʵ���֮��4�U5��10Fe2����6Br����8Cl2��10Fe3����3Br2��16Cl�� |
D��̼�������Һ�м���������ʯ��ˮ��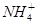 �� ��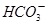 ��Ca2����2OH����CaCO3����NH3��H2O��H2O ��Ca2����2OH����CaCO3����NH3��H2O��H2O |
������5�ֻ����е�2����ͬ����������ϣ��γɵ��л��������ڵ���ʵ��� )
�١�OH �ڡ�CH3 �ۡ�COOH ��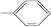 �ݡ�CHO
�ݡ�CHO
| A��3�� | B��4�� | C��5�� | D��6�� |
���л�ѧ����ʽ�У����������ӷ���ʽBa2++SO42- =BaSO4����ʾ����
| A��Ba(NO3)2+H2SO4 = BaSO4��+2HNO3 |
| B��BaCl2+Na2SO4=BaSO4��+2NaCl |
| C��BaCO3+H2SO4=BaSO4��+H2O+CO2�� |
| D��BaCl2+H2SO4=BaSO4��+2HCl |
���������У������ڵ���ʵ���
| A������ | B��NaCl | C��NaOH | D��H2SO4 |
�����й����ӵ�˵����ȷ����
| A��H+��NO3����Fe2+��Na+������������Һ���ܹ��������� |
| B��Cl2ͨ��ˮ�е����ӷ���ʽ��Cl2+H2O��2H++Cl��+ClO�� |
| C��1mol Na2O2�к���2mol�����Ӻ�1mol������ |
| D����ij��Һ�м������ʯ��ˮ����ǣ��ټ������ᣬ�����������ɫ��ζ�����������ԭ��Һ��һ������̼������� |
�������ӷ���ʽ��д��ȷ����
| A����Fe(OH)3�����м���HI��Һ��Fe(OH)3 +3H+=Fe3+ +3H2O |
| B����ϡ��ˮ��ͨ������SO2��NH3?H2O+SO2=NH4++HSO3- |
| C�������ʯ��ˮ�м�������NaHCO3��Һ�� Ca2+ +OH-+HCO3-=CaCO3��+ H2O |
| D��������KMnO4��Һ�м���H2O2�� 2MnO4-+5H2O2 +6H+=2Mn2++5O2��+ 8H2O |