��Ŀ����
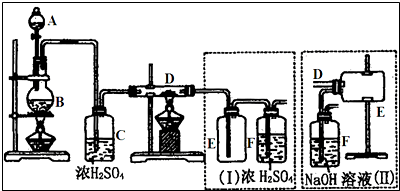
16����ͼ��ʾ��ʵ��װ�ÿ������ⶨ������Ԫ�ص�ij������X�ķ���ʽ��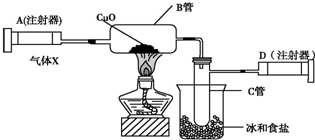
��ע����A��װ��240mL����X��������ͨ��������������װ�к�������ͭ�IJ�����B��ʹ֮��ȫ��Ӧ��ʵ����Ϊ��B���еĺ�ɫ��ĩ��ɺ�ɫ��ĩ��ʵ��ǰB����20.32g��ʵ���B����20.00g����C�����ռ���ˮ����ע����D���ռ���������
��1������X����N��HԪ����ɵģ�
��2����֪��ʵ��ʱ���¶Ⱥ�ѹǿ�£�1mol����X�������24000mL����ע����A��240mL����X��ȫ��Ӧ����ע����D�еõ��ĵ���Ϊ0.28g����X��Ħ������Ϊ32g•mol-1��
��3��ͨ�����㣬ȷ������X�ķ���ʽΪN2H4��
��4��д��B�з�����Ӧ�Ļ�ѧ����ʽ������X�ڸ������²������ֽⷴӦ��2CuO+N2H4$\frac{\underline{\;\;��\;\;}}{\;}$2Cu+2H2O+N2��
���� ��1������Ŀ��Ϣ��֪��C�����ռ���ˮ��D���ռ���N2��˵������X������ͭ��Ӧ�е�����ˮ���ɣ�����xֻ��������Ԫ�أ�����Ԫ���غ��֪������X����N��HԪ�أ�
��2��1molX������������24000mL����240mLX��������ʵ���Ϊ0.01mol����Ӧ�ռ���������������0.28g�����ʵ���Ϊ0.01mol��B����CuO��O��20.32 g-20.00 g=0.32 g�����ʵ���Ϊ0.02mol�������ɵ�ˮ��HԪ�ص�����Ϊ0.04mol��1g/mol=0.04g����������X������Ϊ0.28g+0.04g=0.32g���ٸ���Ħ���������ڼ�������X��Ħ��������
��3�����ݣ�2���п�֪����X�����ʵ��������е�N��Hԭ�ӵ����ʵ������ݴ���д����X�ķ���ʽ��
��4������Ŀ��Ϣ��֪��B���еĺ�ɫ��ĩ��ɺ�ɫ��ĩ��˵����Ӧ����Cu��C�����ռ���ˮ��D���ռ���N2��˵������X������ͭ��Ӧ�е�����ˮ���ɣ�������X������ͭ��Ӧ����ͭ��������ˮ��
��� �⣺��1������Ŀ��Ϣ��֪��C�����ռ���ˮ��D���ռ���N2��˵������X������ͭ��Ӧ�е�����ˮ���ɣ�����xֻ��������Ԫ�أ�����Ԫ���غ��֪������X����N��HԪ�أ�
�ʴ�Ϊ��N��H��
��2��1molX������������24000mL����240mLX��������ʵ���Ϊ$\frac{240ml}{24000ml}$��1mol=0.01mol����Ӧ�ռ���������������0.28g�����ʵ���Ϊ$\frac{0.28g}{28g/mol}$=0.01mol��B����CuO��O��20.32 g-20.00 g=0.32 g�����ʵ���Ϊ$\frac{0.32g}{16g/mol}$=0.02mol��n��H2O��=0.02mol�������ɵ�ˮ��HԪ�ص�����Ϊ0.02 mol��2��1g/mol=0.04g����������X������Ϊ0.28g+0.04g=0.32g��������X��Ħ������Ϊ$\frac{0.32g}{0.01mol}$=32g/mol���ʴ�Ϊ��32��
��3�����ݣ�2���п�֪n��X��=0.01 mol��n��N��=0.01mol��2=0.02mol��n��H��=0.02 mol��2=0.04mol�����n��X����n��N����n��H��=0.01 mol��0.02 mol��0.04mol=1��2��4������X�Ļ�ѧʽΪN2H4���ʴ�Ϊ��N2H4��
��4������Ŀ��Ϣ��֪��B���еĺ�ɫ��ĩ��ɺ�ɫ��ĩ��˵����Ӧ����Cu��C�����ռ���ˮ��D���ռ���N2��˵������X������ͭ��Ӧ�е�����ˮ���ɣ�������X������ͭ��Ӧ����ͭ��������ˮ����Ӧ����ʽΪ2CuO+N2H4$\frac{\underline{\;\;��\;\;}}{\;}$2Cu+2H2O+N2���ʴ�Ϊ��2CuO+N2H4$\frac{\underline{\;\;��\;\;}}{\;}$2Cu+2H2O+N2��
���� ���⿼��������ɵ��ƶϵȣ��Ǽ������ƶϣ��Ѷ��еȣ����������غ�����ƶ��ǽ���ĸ���

 �߽�������ϵ�д�
�߽�������ϵ�д�| A�� | ���к͵ζ�ʵ���У��ζ���������ˮϴ�Ӻ����ñ�Һ��ϴ���ټӽ���Һ | |
| B�� | ����50mL 0.55mo1•L-1������������Һ���ֱ���50mL 0.50mo1•L-1������� 50mL0.50mo1•L-1�������ַ�Ӧ������Ӧ�ⶨ���к��Ȳ���� | |
| C�� | ��ͼװ����ʾ����ȷ�ⶨ�к���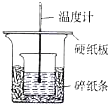 | |
| D�� | �����к͵ζ�����ʱ����������ƿ������ת����������Һ�����٣��۾�Ҫʼ��ע�ӵζ�������ҺҺ��ı仯 |
| A�� | ����ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| B�� | Na2O2 ����ˮ����O2��Na2O2+H2O�T2Na++2OH-+O2�� | |
| C�� | ����ʯ���ڴ��CaCO3+2H+�TCa2++CO2��+H2O | |
| D�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O |
| A�� | 4�� | B�� | 5�� | C�� | 6�� | D�� | 7�� |
| A�� |  ���Ÿ���IJ���Ƭ���� | B�� |  Ũ���ḯʴֽ�� | ||
| C�� |  �úϽ��ڴ������Ͽ̻� | D�� |  ���������� |