��Ŀ����
����Ŀ���ס��Ҷ��Ƕ�Ԫ���廯�����32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������![]() ��Һ�����ˡ�ϴ�ӡ�����ó���
��Һ�����ˡ�ϴ�ӡ�����ó���![]() ����Һ���ٵμ�NaOH��Һ���ֳ�����ɫ������
����Һ���ٵμ�NaOH��Һ���ֳ�����ɫ������
���ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�������ң�����ϡ����ʹ����ȫ�ܽ⣬��Һ��ΪA��B���ȷ֣���A�м�������NaOH��Һ�����ˡ�ϴ�ӡ����յõ�����ɫ����28g���������������ɫ�������Ԫ����ͬ����B�м���![]() ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ�����ù���
ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ�����ù���![]() ��
��
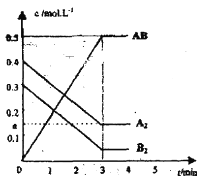
![]() д�����ɼ������ӵĽṹʾ��ͼ______��32g��������Ũ�����з�Ӧת�Ƶĵ�����Ϊ______��
д�����ɼ������ӵĽṹʾ��ͼ______��32g��������Ũ�����з�Ӧת�Ƶĵ�����Ϊ______��
![]() �ҵĻ�ѧʽ______��ϡ�����ܽ��ҵĻ�ѧ����ʽ______��
�ҵĻ�ѧʽ______��ϡ�����ܽ��ҵĻ�ѧ����ʽ______��
![]() ���������������г�����յ��������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽΪ______�����ʵ��֤���˲���Ӧ�����Һ�н���Ԫ�صĻ��ϼ�______��
���������������г�����յ��������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽΪ______�����ʵ��֤���˲���Ӧ�����Һ�н���Ԫ�صĻ��ϼ�______��
���𰸡�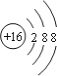
![]()
![]()
![]()
![]() ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��
ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��![]() ��Һ������Һ��ɫ��ԭ��
��Һ������Һ��ɫ��ԭ��![]() ���ӡ�����һ���м���KSCN��Һ��������Ѫ��ɫ��Һ����ԭ��
���ӡ�����һ���м���KSCN��Һ��������Ѫ��ɫ��Һ����ԭ��![]() ��������
��������
��������
��32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������![]() ��Һ�����ˡ�ϴ�ӡ�����ó���
��Һ�����ˡ�ϴ�ӡ�����ó���![]() ���ó���Ϊ���ᱵ�������ʵ���Ϊ��
���ó���Ϊ���ᱵ�������ʵ���Ϊ��![]() �����������غ��֪���к���
�����������غ��֪���к���![]() ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��
ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��![]() ����Ļ�ѧʽΪ��
����Ļ�ѧʽΪ��![]() ��
��
���ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�����ң�����ϡ����ʹ��ȫ���ܽ⣬��Һ��ΪA��B���ȷݣ���A�м�����������������Һ�����ˡ�ϴ�ӡ����յú���ɫ����28g���ú���ɫ����Ϊ![]() ��������������Ԫ�ص����ʵ���Ϊ�
��������������Ԫ�ص����ʵ���Ϊ�![]() ������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���
������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���![]() ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����
ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����![]() ����Ӧ����ͭ�����ʵ���Ϊ��
����Ӧ����ͭ�����ʵ���Ϊ��![]() �����ݷ�Ӧ
�����ݷ�Ӧ![]() ��֪��
��֪��![]() ͭ��ȫ��Ӧ����
ͭ��ȫ��Ӧ����![]() �������������ᷴӦ���ɵ�Ϊ
�������������ᷴӦ���ɵ�Ϊ![]() ��
��![]() ������ƽ�����ϼ�Ϊ��
������ƽ�����ϼ�Ϊ��![]() �����ҵĻ�ѧʽΪ��
�����ҵĻ�ѧʽΪ��![]() ��
��
![]() ��Ϊ
��Ϊ![]() ������������Ϊ�����ӣ������ӽṹʾ��ͼΪ��
������������Ϊ�����ӣ������ӽṹʾ��ͼΪ�� ��
��![]() �����ʵ���Ϊ��
�����ʵ���Ϊ��![]() ��Ũ����������CuԪ�ش�
��Ũ����������CuԪ�ش�![]() ת����
ת����![]() �ۡ���Ԫ�ش�
�ۡ���Ԫ�ش�![]() ת����
ת����![]() �ۣ���Ӧ��ʧȥ���������ʵ���Ϊ��
�ۣ���Ӧ��ʧȥ���������ʵ���Ϊ��![]() ����Ӧת�Ƶ�������Ϊ
����Ӧת�Ƶ�������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��![]() ��
��
![]() ���ݷ�����֪���ҵĻ�ѧʽΪ��
���ݷ�����֪���ҵĻ�ѧʽΪ��![]() ��ϡ�������ҷ�Ӧ�Ļ�ѧ����ʽΪ��
��ϡ�������ҷ�Ӧ�Ļ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
![]() �������������г�����յ��������Ϊ����������������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽ��
�������������г�����յ��������Ϊ����������������ͨ��һ����A��Һ�У��÷�Ӧ�����ӷ���ʽ��![]() ���÷�Ӧ����Һ�к����������ӣ������������ӵķ���Ϊ��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��
���÷�Ӧ����Һ�к����������ӣ������������ӵķ���Ϊ��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��![]() ��Һ������ɫ������
��Һ������ɫ������![]() ����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������
����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������![]() �������ʴ�Ϊ��
�������ʴ�Ϊ��![]() ��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��
��ȡ��Ӧ�����Һ�������Թ��У���һ���м����ữ��![]() ��Һ������ɫ������
��Һ������ɫ������![]() ����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������
����������һ���м���KSCN��Һ��������Ѫ��ɫ��Һ������![]() ��������32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������
��������32g�ķ�ĩ��������Ũ���Ტ���ȣ���ȫ�ܽ����ɫ��Һ�������Һ�м�������![]() ��Һ�����ˡ�ϴ�ӡ�����ó���
��Һ�����ˡ�ϴ�ӡ�����ó���![]() ���ó���Ϊ���ᱵ�������ʵ���Ϊ��
���ó���Ϊ���ᱵ�������ʵ���Ϊ��![]() �����������غ��֪���к���
�����������غ��֪���к���![]() ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��
ԭ�ӣ���Һ���ٵμ�NaOH��Һ���ֳ�����ɫ����������ɫ����Ϊ������ͭ��˵�����к���Cu���ӣ�32g���к���ͭԪ�ص����ʵ���Ϊ��![]() ����Ļ�ѧʽΪ��
����Ļ�ѧʽΪ��![]() �����ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�����ң�����ϡ����ʹ��ȫ���ܽ⣬��Һ��ΪA��B���ȷݣ���A�м�����������������Һ�����ˡ�ϴ�ӡ����յú���ɫ����28g���ú���ɫ����Ϊ
�����ҵĿ�ʯ��Ȼ���д����϶࣬��ȡһ�����ң�����ϡ����ʹ��ȫ���ܽ⣬��Һ��ΪA��B���ȷݣ���A�м�����������������Һ�����ˡ�ϴ�ӡ����յú���ɫ����28g���ú���ɫ����Ϊ![]() ��������������Ԫ�ص����ʵ���Ϊ�
��������������Ԫ�ص����ʵ���Ϊ�![]() ������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���
������Ϊ�����Ӻ��������ӵĻ����������������ɫ��������Ԫ����ͬ�������к���Fe��OԪ�أ���B�м���![]() ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����
ͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����![]() ����Ӧ����ͭ�����ʵ���Ϊ��
����Ӧ����ͭ�����ʵ���Ϊ��![]() �����ݷ�Ӧ
�����ݷ�Ӧ![]() ��֪��
��֪��![]() ͭ��ȫ��Ӧ����
ͭ��ȫ��Ӧ����![]() �������������ᷴӦ���ɵ�Ϊ
�������������ᷴӦ���ɵ�Ϊ![]() ��
��![]() ������ƽ�����ϼ�Ϊ��
������ƽ�����ϼ�Ϊ��![]() �����ҵĻ�ѧʽΪ��
�����ҵĻ�ѧʽΪ��![]() ���ݴ˷������
���ݴ˷������

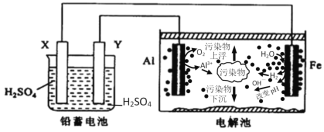
����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ������Cr(III)�Ĵ���������������:

��֪: �� �����ȡҺ�еĽ���������Ҫ��Cr3+�������Fe2+��Al3+��Ca2+��Mg2+��
�� Cr2O72-+H2O![]() 2CrO42-+2H+
2CrO42-+2H+
�� �����£�����������������������ʽ����ʱ��Һ��pH����:
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
������ȫʱ��pH | 3.2 | 11.1 | 5.4(>8�ܽ�) | 9(>9�ܽ�) |
(1)ʵ������18.4mol/L��Ũ��������500mL2mol/L������ʱ������Ͳ���ձ��Ͳ������⣬�����õ��IJ���������_________________________��
(2)H2O2�������ǽ���ҺI�е�Cr3+ת��ΪCr2O72-,д���˷�Ӧ�����ӷ���ʽ__________��
(3)����II�����õ���������ҪΪ______(�ѧʽ)��
(4)�����ӽ�����֬�ķ�Ӧԭ��Ϊ:Mn++nNaR=MRn+nNa+,�����������ӽ�����֬�ɳ�ȥ��ҺII�еĽ�����������_________________��
(5)���������У�ÿ��ԭ1molCrO42��ʱ����Ҫ����SO2 _______ mol��