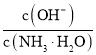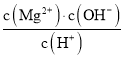题目内容
【题目】草酸亚铁晶体(FeC2O4·2H2O)常用做分析试剂及显影剂等。下图是将一定质量的草酸亚铁在氩气气氛中进行热重分析示意图(TG%表示残留固体质量占原样品总质量的百分数)。

请回答下列问题:
(l)B处时残留物的化学式为___________。
(2)A→C整个反应过程中总反应的化学方程式为___________。
(3)取上述分解得到的600℃时的固体放在空气中继续加热时其质量增加,发生反应的化学方程式为___________。由此说明上述FeC2O4·2H2O在氩气气氛中进行热重分析的原因是___________。
(4)若将分解得到的600℃时的固体与足量的稀硫酸反应后,将溶液浓缩、冷却,有带7个结晶水的晶体析出,该晶体的化学式为__________,该晶体与草酸亚铁晶体分解有类似,得到四种氧化物且物质的量之比为1:1:1:14,则该化学方程式为__________。
【答案】FeC2O4 FeC2O4·2H2O![]() FeO +CO↑+CO2↑+2H2O 6FeO+O2
FeO +CO↑+CO2↑+2H2O 6FeO+O2![]() 2Fe3O4 防止FeO在加热过程中被空中的氧气氧化 FeSO4·7H2O 2FeSO4·7H2O
2Fe3O4 防止FeO在加热过程中被空中的氧气氧化 FeSO4·7H2O 2FeSO4·7H2O![]() Fe2O3+SO2↑+SO3↑+14H2O
Fe2O3+SO2↑+SO3↑+14H2O
【解析】
(1)FeC2O4·2H2O中H2O%=![]() =20%,刚好在B点的TG%=80%,则B点表示所有的结晶水都完全失去,得到的固体成分为FeC2O4;故答案为:FeC2O4;
=20%,刚好在B点的TG%=80%,则B点表示所有的结晶水都完全失去,得到的固体成分为FeC2O4;故答案为:FeC2O4;
(2)当TG%=40%时,得到应该是铁的氧化物,则有![]() =0.4,x=1,则变化过程中铁的化合价没改变,只有碳的化合价发生变化,则A→C整个反应过程中总反应的化学方程式为FeC2O4·2H2O
=0.4,x=1,则变化过程中铁的化合价没改变,只有碳的化合价发生变化,则A→C整个反应过程中总反应的化学方程式为FeC2O4·2H2O![]() FeO +CO↑+CO2↑+2H2O;故答案为:FeC2O4·2H2O
FeO +CO↑+CO2↑+2H2O;故答案为:FeC2O4·2H2O![]() FeO +CO↑+CO2↑+2H2O;
FeO +CO↑+CO2↑+2H2O;
(3)当分解得到的600℃时的固体FeO在空气中与氧气反应,得到稳定的Fe3O4,因此FeC2O4·2H2O在氩气气氛中进行热重分析的原因防止FeO在加热过程中被空中的氧气氧化;故答案为:6FeO+O2![]() 2Fe3O4;防止FeO在加热过程中被空中的氧气氧化;
2Fe3O4;防止FeO在加热过程中被空中的氧气氧化;
(4)FeO与足量的稀硫酸反应得到的是FeSO4,为了防止其Fe2+水解和Fe2+被氧化,要加适量的硫酸和铁粉,才能得到FeSO4·7H2O,晶体与草酸亚铁晶体分解有类似,得到四种氧化物且物质的量之比为1:1:1:14,则有Fe2O3、SO2、SO3、H2O;故答案为:FeSO4·7H2O;2FeSO4·7H2O![]() Fe2O3+SO2↑+SO3↑+14H2O。
Fe2O3+SO2↑+SO3↑+14H2O。