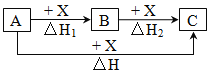��Ŀ����
����Ŀ��A��B��D��E��G��M����Ԫ��λ��Ԫ�����ڱ�ǰ�����ڣ�ԭ�����������������У�Ԫ��A��һ�ֺ��������ӣ�B�ĵ��ʼ��з��Ӿ�������ԭ�Ӿ��壬������DE2Ϊ����ɫ���壬G��ǰ�������е縺����С��Ԫ�أ�M��ԭ�Ӻ����������G��10��
��ش��������⣺
��1����̬Gԭ�ӵĺ�������Ų�ʽ��________��M��Ԫ�����ڱ��е�λ����_______��Ԫ��B��D��E�ĵ�һ�������ɴ�С��˳��Ϊ______________����Ԫ�ط��ű�ʾ����
��2��Ԫ��A��E��ɵ������ӿռ乹��Ϊ________��������ABD�ĽṹʽΪ______������Bԭ�ӵ��ӻ���ʽΪ________��
<span style="font-size: 15px; font-family: "����";"><span contenteditable="true">��3��</span></span>D������������Ӧ��ˮ�����������DA3�����������ӻ������ң������£����ס�������Һ��pH������5������ˮ�������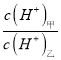 =_________������Һ���������ӵ����ʵ���Ũ���ɴ�С��˳����_______________________________��
=_________������Һ���������ӵ����ʵ���Ũ���ɴ�С��˳����_______________________________��
��4��Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2��Ԫ��Y��̬ԭ�ӵ�3p�������4�����ӡ�
��X��Y���γɻ�������X�Ļ��ϼ۵�������������Y�ﵽ8���ӵ��ȶ��ṹ��û�����Ļ�ѧʽΪ______________��
��E���⻯�H2E�����Ҵ��е��ܽ�ȴ���H2Y����ԭ����_________________��
��X���Ȼ����백ˮ��Ӧ���γ������[X(NH3)4]Cl2��1mol��������к��Ц� ������ĿΪ___________��
���𰸡� 1s22s22p5 �������ڵڢ�B �� N>O>C ������ H-C��N SP 10-4 c(NO3-)>c(NH4+)>c(H+)>c(OH-) ZnS ˮ�������Ҵ����Ӽ��γ����������H2S�����γ���� 16NA �� 16��6.02��1023��9.63��1024
��������A��B��D��E��G��M����Ԫ��λ��Ԫ�����ڱ�ǰ�����ڣ�ԭ������������������Ԫ��A��һ�ֺ��������ӣ���AΪHԪ�أ�B�ĵ��ʼ��з��Ӿ�������ԭ�Ӿ��壬��BΪ̼Ԫ�أ�������DE2Ϊ����ɫ���壬��DΪNԪ�ء�EΪOԪ�أ�G��ǰ�������е縺����С��Ԫ�أ���GΪKԪ�أ�M��ԭ�Ӻ����������G��10����MΪCu��
��1����̬Kԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p64s1��MΪCu����Ԫ�����ڱ��е�λ���ǵ������ڵ���B�壬ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�صģ��ʵ�һ�����ܣ�N��O��C��
��2��Ԫ��A��E��ɵ�������ΪH3O+��Oԭ�Ӻ��йµ��Ӷ�Ϊ1���۲���Ӷ���Ϊ3+1=4����Ϊ�����νṹ��������HCN�ĽṹʽΪH-C��N������Cԭ���ӻ������ĿΪ2���ӻ���ʽΪsp�ӻ���
��3��D������������Ӧ��ˮ�����Ϊ���ᣬ����������DA3�����������ӻ�������Ϊ����泥�����������������������ˮ�ĵ��룬�������笠�����ˮ��ٽ���ˮ�ĵ��룬�����£�������Һ��ˮ����������ӵ�����Һ����������Ũ��Ϊ�� ![]() mol/L=10-9mol/L���������Һ��������Ϊˮ���������Ũ��Ϊ10-5mol/L������ˮ�������
mol/L=10-9mol/L���������Һ��������Ϊˮ���������Ũ��Ϊ10-5mol/L������ˮ�������![]() =10-4���������Һ�У�笠�����ˮ�⣬��Һ�����ԣ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳����c��NO3-����c��NH4+����c��H+����c��OH-����
=10-4���������Һ�У�笠�����ˮ�⣬��Һ�����ԣ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳����c��NO3-����c��NH4+����c��H+����c��OH-����
��5��Ԫ��Xλ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ�������������Ϊ2�����������Ϊ2+8+18+2=30����XΪZn��Ԫ��Y��̬ԭ�ӵ�3p�������4�����ӣ���YΪSԪ�أ�
��X��Y���γɻ�������X�Ļ��ϼ۵�������������Znλ����B�壬���仯�ϼ�Ϊ+2�ۣ�Y�ﵽ8���ӵ��ȶ��ṹʱ�Ļ��ϼ�Ϊ-2�ۣ�������γɵĻ�����ΪZnS��
��ˮ�������Ҵ�����֮���γ������H2O���Ҵ��е��ܽ�ȴ���H2S��
��X���Ȼ����백ˮ��Ӧ���γ������[Zn��NH3��4]Cl2��1mol��������к���12molN-H��4mol��λ�����ʺ����� ������ĿΪ16NA��

����Ŀ��Ϊ���ᴿ�±���������(������Ϊ����)���йس����Լ��ͷ��뷽����ѡ�����ȷ���ǣ� ��
��� | ���ᴿ������ | �����Լ� | ���뷽�� |
A | ����(��ϩ) | KMnO4������Һ | ϴ�� |
B | ������������ | ˮ | ��Һ |
C | ��(����) | Ũ��ˮ | ���� |
D | ��������(����) | ����̼������Һ | ��Һ |
A. AB. BC. CD. D
����Ŀ��25 ��ʱ����������ĵ��볣�����£�
���ữѧʽ | HNO2 | CH3COOH | HCN | H2CO3 |
���볣�� | 5.1��10-4 | 1.8��10-5 | 6.2��10-10 | K1=4.4��10-7 K2=4.7��10-11 |
��1�������ϱ�������գ�
�����ʵ���Ũ����ͬ�������ᣬ��pH�ɴ�С��˳����___________ ���û�ѧʽ��д����
�ڷֱ�����������ͬpH��HCl��Һ��CH3COOH��Һ�м���������Zn�ۣ���Ӧ�տ�ʼʱ����H2�����ʣ�v(HCl)______v(CH3COOH)���������������������ͬ������Ӧ��ȫ������������������m(H2)����_______m(H2)������
�۽�0.2 mol/L HCN��Һ��0.1 mol/L Na2CO3��Һ�������ϣ�������Ӧ�Ļ�ѧ����ʽΪ___________________________��
��2�������Ϊ10 mL��pH��Ϊ2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000 mL��ϡ��������ҺpH�仯��ͼ��ʾ��ϡ�ͺ�HX��Һ��ˮ�����c(H��)___������Һ��ˮ�����c(H��) �����볣��Ka(HX)___Ka(CH3COOH)�����������������������

��3��CO32- �� NO2- ��CN-��CH3COO-���H+��������ǿ������˳��Ϊ_______________________�������ӷ��ű�ʾ��