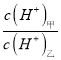��Ŀ����
����Ŀ��25 ��ʱ����������ĵ��볣�����£�
���ữѧʽ | HNO2 | CH3COOH | HCN | H2CO3 |
���볣�� | 5.1��10-4 | 1.8��10-5 | 6.2��10-10 | K1=4.4��10-7 K2=4.7��10-11 |
��1�������ϱ�������գ�
�����ʵ���Ũ����ͬ�������ᣬ��pH�ɴ�С��˳����___________ ���û�ѧʽ��д����
�ڷֱ�����������ͬpH��HCl��Һ��CH3COOH��Һ�м���������Zn�ۣ���Ӧ�տ�ʼʱ����H2�����ʣ�v(HCl)______v(CH3COOH)���������������������ͬ������Ӧ��ȫ������������������m(H2)����_______m(H2)������
�۽�0.2 mol/L HCN��Һ��0.1 mol/L Na2CO3��Һ�������ϣ�������Ӧ�Ļ�ѧ����ʽΪ___________________________��
��2�������Ϊ10 mL��pH��Ϊ2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000 mL��ϡ��������ҺpH�仯��ͼ��ʾ��ϡ�ͺ�HX��Һ��ˮ�����c(H��)___������Һ��ˮ�����c(H��) �����볣��Ka(HX)___Ka(CH3COOH)�����������������������

��3��CO32- �� NO2- ��CN-��CH3COO-���H+��������ǿ������˳��Ϊ_______________________�������ӷ��ű�ʾ��
���𰸡� HCN > H2CO3 > CH3COOH > HNO2 = < HCN + Na2CO3 = NaCN + NaHCO3 > > CO32- > CN- > CH3COO- > NO2-
����������1�����ݵ���ƽ�ⳣ���Ĵ�С����������ƽ�ⳣ��Խ��������Խǿ�����ԣ�HCN��H2CO3��CH3COOH��HNO2������Խǿ����pHԽС������pH��ϵΪ��HCN��H2CO3��CH3COOH��HNO2��
��pH��ͬ�IJ�ͬ�����У�������Ũ����ͬ����Zn��Ӧ������ͬ�����������Ũ�ȴ���������Ũ�ȣ����������Ũ�ȵ���������Ũ�ȣ����Դ����Ũ�ȴ���HCl��Ũ�ȣ���������������������m��H2��������m��H2��������
���ɵ��볣����֪�����ԣ�H2CO3��HCN��CO3-����0.2 mol/L HCN��Һ��0.1 mol/L Na2CO3��Һ�������ϣ�������Ӧ�Ļ�ѧ����ʽΪHCN+Na2CO3�TNaCN+NaHCO3��
��2����ͼ��֪��ϡ�ͺ�HX�������ɵ�c��H+��С����ˮ�ĵ�����������С������HX��Һ��ˮ���������c��H+����ϡ����ͬ��������ǿ����pH�仯�ϴ�ǿ������볣���ϴ���ͼ��֪ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
��3����Խ�����������Խ��ˮ�⣬��������ӵ�����Խǿ����CO32- �� NO2- ��CN-��CH3COO-���H+��������ǿ������˳��ΪCO32-��CN-�� CH3COO-��NO2-��

����Ŀ��Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ�鷽������̽����
��1��ȡ�����ʵ���Ũ�ȡ��������H2O2��Һ�ֱ����H2O2�ķֽ�ʵ�飬ʵ�鱨�����±���ʾ������ͽ����ԣ���
��� | �¶�/�� | ���� | ���� | ���� |
1 | 40 | FeCl3��Һ | ||
2 | 20 | FeCl3��Һ | ||
3 | 20 | MnO2 | ||
4 | 20 | �� |
��ʵ��1��2�о�����________________��H2O2�ֽ����ʵ�Ӱ�졣
��ʵ��2��3��Ŀ����_______________��
��2��������֪��Cu2����H2O2�ֽ�Ҳ�д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч������С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺

�ٶ��Է�������ͼ��ͨ���۲�________________�����ԱȽϵó����ۡ���ͬѧ�����CuSO4��Һ��ΪCuCl2��Һ����������������_______________��
�ڶ�����������ͼ����ʾ��ʵ��ʱ���ռ���40 mL����Ϊ��������������Ӱ��ʵ������أ�ʵ������Ҫ������������_____________��
��3�����Ը��������Һ�Ͳ�����Һ�ɷ�����Ӧ��2KMnO4��5H2C2O4��3H2SO4=K2SO4��2MnSO4��8H2O��10CO2����ʵ��ʱ���ֿ�ʼ��Ӧ���ʽ�������Һ��ɫ�����ԣ���һ��ʱ���ͻȻ��ɫ����Ӧ�������Լӿ졣�Դ�չ�����ۣ�
��ijͬѧ��ΪKMnO4��H2C2O4�ķ�Ӧ�� �ȷ�Ӧ������_______________��
�ڴ�Ӱ�컯ѧ��Ӧ���ʵ����ؿ�������Ϊ��������____________________��Ӱ�졣Ҫ֤����IJ��룬ʵ�鷽���� ��