��Ŀ����
ij�о���ѧϰС�鰴��ͼ��ʾװ�ý���̽��ʵ�顣
��ش��������⣺
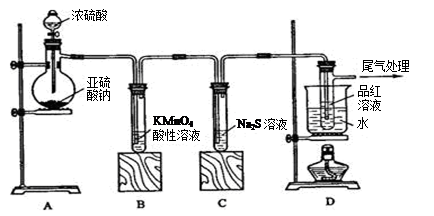
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������У�װ��B������������ ��������Ӧ�����ӷ���ʽΪ ��
��3��װ��C�з����������� ����˵��SO2���е������� ��
��4��װ��D��Ŀ����̽��SO2��Ʒ�����õ��ȶ��ԣ���д��ʵ�����������
��
��5�������ʵ����֤װ��B��Һ�Ƿ���SO42�� ��
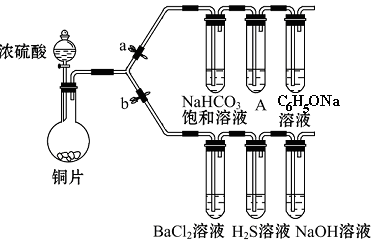
��6�����ڿ�ͼ�ڻ���SO2β������װ��ͼ��


��ش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������У�װ��B������������ ��������Ӧ�����ӷ���ʽΪ ��
��3��װ��C�з����������� ����˵��SO2���е������� ��
��4��װ��D��Ŀ����̽��SO2��Ʒ�����õ��ȶ��ԣ���д��ʵ�����������
��
��5�������ʵ����֤װ��B��Һ�Ƿ���SO42�� ��
��6�����ڿ�ͼ�ڻ���SO2β������װ��ͼ��

��1�� Na2SO3+H2SO4��Ũ����Na2SO4+SO2��+H2O ��2�֣�
��2����Һ���Ϻ�ɫ��1�֣���Ϊ��ɫ��1�֣����Ϻ�ɫ���Ը��������ɫ
5SO2+2MnO4��+2H2O��5SO42��+2Mn2++4H+��2�֣�
��3����ɫ��Һ���ֻ�ɫ��1�֣����ǻ���� ��1�֣� �����ԣ�1�֣�
��4��Ʒ����Һ��ɫ�رշ�Һ©��������1�֣�����ȼ�ƾ��Ƽ��ȣ�1�֣�����Һ�ָ���ɫ��1�֣�
��ȡ������ɫ��Ʒ����Һ���Թܣ�1�֣������ȣ�1�֣�����Һ�ָ���ɫ��1�֣�
��5��ȡ��������Һ���μ��������Ȼ�����Һ�����а�ɫ����������˵������SO42����������������ȫ�Ե�1�֣������ް�ɫ����������˵��û��SO42����������ȫ�Ե�1�֣��������ȼ�������ϡ�����ữ�������۷֣�
��6�� ��2�֣�����װ��1�֣���ע�Լ�1�֣�װ�á��Լ�
��2�֣�����װ��1�֣���ע�Լ�1�֣�װ�á��Լ�
��2����Һ���Ϻ�ɫ��1�֣���Ϊ��ɫ��1�֣����Ϻ�ɫ���Ը��������ɫ
5SO2+2MnO4��+2H2O��5SO42��+2Mn2++4H+��2�֣�
��3����ɫ��Һ���ֻ�ɫ��1�֣����ǻ���� ��1�֣� �����ԣ�1�֣�
��4��Ʒ����Һ��ɫ�رշ�Һ©��������1�֣�����ȼ�ƾ��Ƽ��ȣ�1�֣�����Һ�ָ���ɫ��1�֣�
��ȡ������ɫ��Ʒ����Һ���Թܣ�1�֣������ȣ�1�֣�����Һ�ָ���ɫ��1�֣�
��5��ȡ��������Һ���μ��������Ȼ�����Һ�����а�ɫ����������˵������SO42����������������ȫ�Ե�1�֣������ް�ɫ����������˵��û��SO42����������ȫ�Ե�1�֣��������ȼ�������ϡ�����ữ�������۷֣�
��6��
 ��2�֣�����װ��1�֣���ע�Լ�1�֣�װ�á��Լ�
��2�֣�����װ��1�֣���ע�Լ�1�֣�װ�á��Լ������������1��Ũ������ǿ�ᣬ���������Ʒ�Ӧ���������ơ�SO2��ˮ�����װ��A���Ʊ�SO2�ģ�����װ��A�з�����Ӧ�Ļ�ѧ����ʽΪNa2SO3+H2SO4��Ũ����Na2SO4+SO2��+H2O��
��2��SO2���л�ԭ�ԣ����Ը��������Һ����ǿ�����ԣ���SO2����������ԭ��Ӧ�����ʵ������У�װ��B��������������Һ���Ϻ�ɫ��Ϊ��ɫ���Ϻ�ɫ���Ը��������ɫ��������Ӧ�����ӷ���ʽΪ5SO2+2MnO4��+2H2O��5SO42��+2Mn2++4H+��
��3��SO2��SԪ�صĻ��ϼ��ǣ�4�ۣ����������ԡ�Na2S��SԪ�صĻ��ϼ��ǣ�2�ۣ����л�ԭ�ԡ����SO2ͨ�뵽Na2S��Һ�з���������ԭ��Ӧ���ɵ���S����������װ��C�з�������������ɫ��Һ���ֻ�ɫ���ǻ��������Ӧ�����ӷ���ʽΪSO2��2S2����2H2O��3S����4OH����
��4������SO2��Ư���Բ��ȶ����ڼ��ȵ��������ָֻ���ԭ������ɫ�����̽��SO2��Ʒ�����õ��ȶ��Ե�ʵ�������������Ʒ����Һ��ɫ�رշ�Һ©����������ȼ�ƾ��Ƽ��ȣ���Һ�ָ���ɫ��ȡ������ɫ��Ʒ����Һ���Թܣ����ȣ���Һ�ָ���ɫ��
��5�����ᱵ�Dz�����ˮҲ��������İ�ɫ�������ݴ˿��Լ���SO42���������֤װ��B��Һ�Ƿ���SO42���ķ�����ȡ��������Һ���μ��������Ȼ�����Һ�����а�ɫ����������˵������SO42�������ް�ɫ����������˵��û��SO42����
��6��SO2�Ǵ�����Ⱦ���Ҫβ������������SO2���������������������������Һ���գ������ȷ��װ��ͼΪ
 ��2�Ʊ���������֤��β�������Լ����Ӽ���
��2�Ʊ���������֤��β�������Լ����Ӽ���
��ϰ��ϵ�д�
�����Ŀ