��Ŀ����
�Ӵ������������Ȱ�SO2��������SO3��Ȼ����Ũ�������յõ���SO3��ȡ��Ʒ��ij������������ʱ������Ӵ��ҵ�ԭ�����ɷ�ΪSO27%��O2 11%��N2 82%�������������
��1�������״����10 m3ԭ�����е�SO2���ʵ���______________mol��
��2�������״����1 0m3ԭ���������� ǧ�ˡ�
��3����SO2��ת����Ϊ99��2%������Ӵ��ҵ�����������SO3��������� ��
��4�����Ӵ��ҵ����������к�6��72���������������SO3���ѳ��������ͽ�����������
98��3%���������գ��ɵõ�������H2SO4����H2SO4��SO3�Ļ������к���������Ϊ20����SO3������������1000 m3�������壨������Ϊ��״��������Ҫ��98��3%����������� ǧ�ˡ�
��1�������״����10 m3ԭ�����е�SO2���ʵ���______________mol��
��2�������״����1 0m3ԭ���������� ǧ�ˡ�
��3����SO2��ת����Ϊ99��2%������Ӵ��ҵ�����������SO3��������� ��
��4�����Ӵ��ҵ����������к�6��72���������������SO3���ѳ��������ͽ�����������
98��3%���������գ��ɵõ�������H2SO4����H2SO4��SO3�Ļ������к���������Ϊ20����SO3������������1000 m3�������壨������Ϊ��״��������Ҫ��98��3%����������� ǧ�ˡ�
��1��31��25��2�֣�
��2��13��82Kg��4�֣�
��3��7��19%��4�֣�
��4��665Kg��6�֣�
��2��13��82Kg��4�֣�
��3��7��19%��4�֣�
��4��665Kg��6�֣�
�����������1��ԭ������SO2���������Ϊ7%����10m3ԭ�����е�SO2�����Ϊ10m3��7%=0��7m3=700L���ʱ�״���¶�����������ʵ���Ϊ
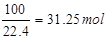 �ʴ�Ϊ��31��25��
�ʴ�Ϊ��31��25����2��ԭ������O2���������Ϊ11%����10m3ԭ�����е�O2�����Ϊ10m3��11%=1��1m3=1100L���ʱ�״�������������ʵ���Ϊ
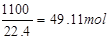 ԭ������N2���������Ϊ82%����10m3ԭ�����е�N2�����Ϊ10m3��82%=8��2m3=8200L���ʱ�״���µ��������ʵ���Ϊ
ԭ������N2���������Ϊ82%����10m3ԭ�����е�N2�����Ϊ10m3��82%=8��2m3=8200L���ʱ�״���µ��������ʵ���Ϊ �ʱ�״����10m3ԭ����������Ϊ��31��25mol��64g/mol+49��11mol��32g/mol+366��07mol��28g/mol=13821��48��13��82kg���𣺱�״����10m3ԭ����������Ϊ13��82kg��
�ʱ�״����10m3ԭ����������Ϊ��31��25mol��64g/mol+49��11mol��32g/mol+366��07mol��28g/mol=13821��48��13��82kg���𣺱�״����10m3ԭ����������Ϊ13��82kg����3��SO2��ת����Ϊ99��2%����μӷ�Ӧ�Ķ�����������Ϊ10m3��7%��99��2%=0��6944m3����
2SO2+O2=2SO3 ������١�V
2 2 1
0��6944m3 0��6944m3 0��3472m3
�ʷ�Ӧ����������Ϊ10m3-0��3472m3=9��6528m3��
�ʽӴ��ҵ�����������SO3�����Ϊ0��6944m3���������Ϊ
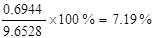
�𣺽Ӵ��ҵ�����������SO3���������Ϊ7��19%��
��4��������ҪŨ���������Ϊmg����Ũ���������������Ϊmg��98g%=0��98mg�����ʵ���Ϊ
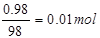 Ũ������ˮ������Ϊmg-0��98mg=0��02mg�����ʵ���Ϊ
Ũ������ˮ������Ϊmg-0��98mg=0��02mg�����ʵ���Ϊ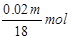 ������������������ɵ���������ʵ���Ϊ
������������������ɵ���������ʵ���Ϊ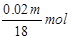 ������������������ʵ���Ϊ0��01m mol+
������������������ʵ���Ϊ0��01m mol+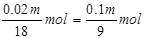 1000m3����������������������Ϊ1000m3��6��72%=67��2m3=67200L��SO3�����ʵ���Ϊ
1000m3����������������������Ϊ1000m3��6��72%=67��2m3=67200L��SO3�����ʵ���Ϊ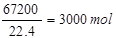 ��ˮ���պ�ʣ���������������ʵ���Ϊ3000mol-
��ˮ���պ�ʣ���������������ʵ���Ϊ3000mol-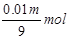
��
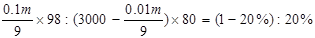 ,���m=664615g=664��6kg��
,���m=664615g=664��6kg��������1000m3������������Ҫ��98%�����������Ϊ664��6kg��

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
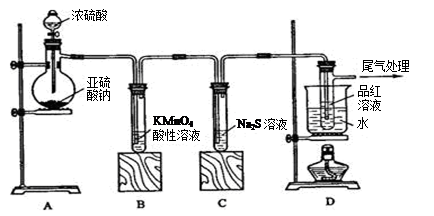

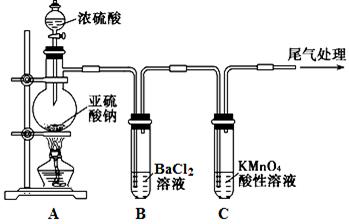
 Na2SO4��SO2����H2O
Na2SO4��SO2����H2O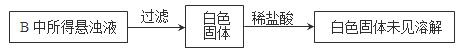
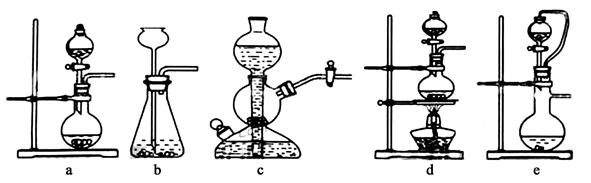
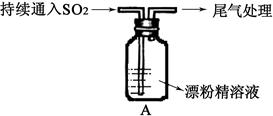
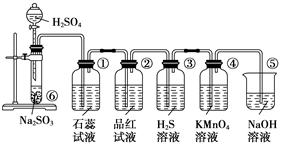
 CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
 [
[