��Ŀ����
17�������£�һԪ��HA��Һ��NaOH��Һ�������ϣ���������仯����ʵ���������±������ж���ȷ���ǣ�������| ʵ���� | ��ʼŨ��/mol•L-1 | ��Ӧ����Һ��pH | |
| c��HA�� | c��NaOH�� | ||
| �� | 0.1 | 0.1 | 9 |
| �� | X | 0.2 | 7 |
| �� | 0.2 | 0.1 | 4.8 |
| �� | 0.1 | 0.2 | y |
| A�� | ʵ��ٷ�Ӧ�����Һ�У�c��HA��ԼΪ$\frac{{K}_{W}}{1��1{0}^{-9}}$mol•L-1 | |
| B�� | ʵ��ڷ�Ӧ�����Һ�У�c��HA����c��Na+��=c��A-����c��H+��=c��OH-�� | |
| C�� | ʵ��۷�Ӧ�����Һ�У�c��HA��+c��H+��=c��OH-��+c��A-�� | |
| D�� | ʵ��ܷ�Ӧ�����Һ�У�c��OH-��-c��H+��-c��HA��=0.05mol•L-1 |
���� A��ʵ����ǵ�Ũ��һԪ��HA��Һ��NaOH��Һ�������ϣ���Ϸ�Ӧ����Һ�ʼ��ԣ�˵���������ᣬ���ɵ�NaA��Һ��A-����ˮ������HA�����������ӣ�HA������������Ũ�Ƚ�����ͬ���������ӻ��������㣻
B����Һ��HA��Һ��NaOH��Һ��Ũ�ȡ����������Լ��ԣ�ʵ��ڷ�Ӧ�����Һ��PH=7��˵����Һ�����ԣ�����Һ��HA�Թ�����X����0.2mol/L����Ũ��HA��NaA�����Һ������ʵ��ۿ�֪��Һ�����ԣ��������ˮ�⣬c��A-����c��HA����
C��ʵ��۵õ���Ũ�ȵ�HA��NaA�����Һ��������Һ�е���غ�������غ���㣬��Һ�е���غ�Ϊ��c��Na+��+c��H+��=c��A-��+c��OH-���������غ�c��A-��+c��HA��=2c��Na+���������������жϣ�
D����ʵ�����Һ�������غ�����������Ӧ����Һ�к��е�Ũ�ȵ�NaA��NaOH������غ�Ϊ��c��Na+��+c��H+��=c��A-��+c��OH-���������غ�Ϊ��c��Na+��=0.05mol/L+c��A-��+c��HA�����������õ���
��� �⣺A��ʵ����ǵ�Ũ��һԪ��HA��Һ��NaOH��Һ�������ϣ���Ϸ�Ӧ����Һ�ʼ��ԣ�˵���������ᣬ���ɵ�NaA��Һ��A-����ˮ������HA�����������ӣ�A-+H2O?HA+OH-��HA������������Ũ�Ƚ�����ͬ���������ӻ���������õ�c��HA��ԼΪ$\frac{{K}_{W}}{1��1{0}^{-9}}$mol•L-1����A��ȷ��
B����Һ��HA��Һ��NaOH��Һ��Ũ�ȡ����������Լ��ԣ�ʵ��ڷ�Ӧ�����Һ��PH=7��˵����Һ�����ԣ�����Һ��HA�Թ�����X����0.2mol/L����Ũ��HA��NaA�����Һ������ʵ��ۿ�֪��Һ�����ԣ��������ˮ�⣬c��A-����c��HA����ʵ��ڷ�Ӧ�����Һ�У�c��Na+��=c��A-����c��HA����c��H+��=c��OH-������C����
C��ʵ��۵õ���Ũ�ȵ�HA��NaA�����Һ����Һ�е���غ�Ϊ��c��Na+��+c��H+��=c��A-��+c��OH-���������غ�c��A-��+c��HA��=2c��Na+������c��HA��+2c��H+��=2c��OH-��+c��A-������C����
D��ʵ�����Һ�к��е�Ũ�ȵ�NaA��NaOH��Ũ�Ⱦ�Ϊ0.05mol/L������غ�Ϊ��c��Na+��+c��H+��=c��A-��+c��OH-���������غ�Ϊ��c��Na+��=0.05mol/L+c��A-��+c��HA�����������õ���c��OH-��-c��H+��-c��HA��=0.05mol•L-1����D��ȷ��
��ѡAD��
���� ���⿼���˵������Һ������Ũ�ȴ�С�Ƚϣ���Һ�е���غ㡢�����غ㡢�����غ�ķ����жϣ����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ���ú˴Ź��������Լ����Ҵ��Ͷ����� | |
| B�� |  ��һ�ȴ�����4�� ��һ�ȴ�����4�� | |
| C�� | ����飨 �������к���4�� �������к���4��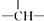 | |
| D�� | �����ϩ ��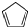 �������������9��ԭ����ͬһƽ���� �������������9��ԭ����ͬһƽ���� |
| A�� | NaHSO3��Һ��c��OH-��=c��HSO3-��+c��H+��+c��H2SO3�� | |
| B�� | CuSO4•��NH4��2SO4•6H2O����Һ�У�c��SO42-����c��NH4+����c��Cu2+����c��H+����C��OH-�� | |
| C�� | ��������ˮ�ﵽƽ��������������������CaCO3���壬$\frac{c��{H}^{+}��}{c��HClO��}$���� | |
| D�� | NaSiO3��Һ��ˮϡ�ͺָ���ԭ�¶ȣ�pH��KW����С |
2KClO3+H2C2O4+H2SO4$\frac{\underline{\;����\;}}{\;}$2ClO2��+K2SO4+2CO2��+2H2O������˵������ȷ���ǣ�������
| A�� | KClO3�ڷ�Ӧ���������� | |
| B�� | 1mol KClO3�μӷ�Ӧ���ڱ�״�����ܵõ�22.4L���� | |
| C�� | �ڷ�Ӧ��H2C2O4�Ȳ���������Ҳ���ǻ�ԭ�� | |
| D�� | 1mol KClO3�μӷ�Ӧ��1mol����ת�� |
| A�� | S��g��+O2��g��=SO2��g����H1��S��s��+O2��g��=SO2��g����H2�����H1����H2 | |
| B�� | NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=-57.4 kJ/mol����20 g NaOH��ϡ��Һ��ϡ�����귴Ӧ���ų�������Ϊ28.7 kJ | |
| C�� | C��ʯī��s��=C�����ʯ��s����H=+1.9 kJ/mol������ʯī��ȡ���ʯ�ķ�Ӧ�����ȷ�Ӧ�����ʯ��ʯī�ȶ� | |
| D�� | 2C��s��+O2��g��=2CO��g����H=-221 kJ/mol����̼��ȼ���ȵ��� 110.5 kJ/mol |
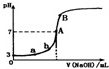
| A�� | ��ͼ��֪HA��һ�����ᣬ��Ka=1.0��10-5 | |
| B�� | ˮ�������������Ũ�ȣ�a��b | |
| C�� | ��NaOH��Һ�����Ϊ10.00mLʱ���У�c��A-��+c��OH-��=c��H+��+c��HA�� | |
| D�� | B����Һ�е�����Ũ�ȹ�ϵΪ��c��Na+����c��A-����c��OH-����c��H+�� |
 ��
��