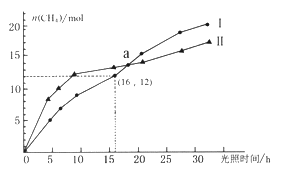
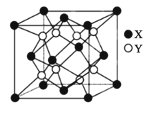
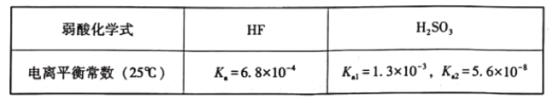
��Ŀ����
����Ŀ���� NA Ϊ�����ӵ�������ֵ������˵����ȷ����
A.25��ʱ��pH��3 �� FeCl3 ��Һ�� H+����ĿΪ 0.001NA
B.��ȩ(HCHO)������Ļ���� 3.0 g�����е�ԭ����Ϊ 0.4NA
C.0.1 mol SO2 �� 1 mol O2 ��ַ�Ӧ��ת�Ƶĵ�����Ϊ 0.2NA
D.��֪����(P4)Ϊ��������ṹ��NA �� P4 �� NA �������������ۼ���Ŀ֮��Ϊ 1��1
���𰸡�B
��������
A��û�и�����Һ���������������Һ�������ӵ���Ŀ����A����
B����ȩHCHO����������ʽ��ΪCH2O����3.0g������к��е���CH2O��ԭ���ŵ����ʵ���Ϊn=![]() =0.1mol�����е�ԭ����Ϊ0.4NA����B��ȷ��
=0.1mol�����е�ԭ����Ϊ0.4NA����B��ȷ��
C��0.1 mol SO2��0.1mol O2�ķ�ӦΪ���淴Ӧ�����ɵ������������ʵ���С��0.1mol��ת�Ƶĵ�����С��0.2NA����C����
D��1mol�������к���6�����ۼ���1mol�����к�4mol���ۼ������ۼ���Ŀ֮��Ϊ3��2����D����
��ѡB��

��ϰ��ϵ�д�
 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
�����Ŀ