��Ŀ����
����Ŀ������(HCOOH)�ǻ�ԭ�����ᣬ��������ҽҩ�ȹ�ҵ������Ҳ�������Ʊ���Ҫ�Ļ���ԭ��[Cu(HCOO)2��4H2O]��
I.��ʽ̼��ͭ���Ʊ�
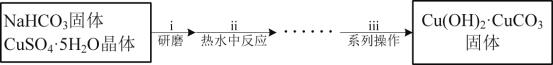
(1)������i������ĥ��Ŀ����__��
(2)������ii�������ķ�Ӧ�ǣ�2CuSO4+4NaHCO3�TCu(OH)2��CuCO3��+3CO2��+2Na2SO4+H2O��ԭ����NaHCO3���������ԭ����__����Ӧ�¶ȵ���80���ԭ����__��
(3)������iii����ϵ�в����������ˡ�__�����
II.������Ʊ�
(4)һ�������£�CO����NaOH���巢����Ӧ��CO+NaOH![]() HCOONa���ٽ�HCOONa�ữ�����ɵ�HCOOH��
HCOONa���ٽ�HCOONa�ữ�����ɵ�HCOOH��
��Ϊ��֤����CO��NaOH���巢���˷�Ӧ������ͬѧ���������֤������ȡ���������������Һ���ڳ����²���pH����pH��7�����֤���÷����Ƿ����__(����������������)�������������ɣ�__��
����ͬѧ�������һ��������֤������ȡ�����������Һ��__(�벹������)��
III.����ͭ�ĺϳɼ����Ȳⶨ
(5)ʵ���Ұ�Cu(OH)2��CuCO3+4HCOOH+5H2O�T2Cu(HCOO)2��4H2O+CO2����Ӧ�Ƶü���ͭ���壬�����²���ⶨ�䴿�ȡ�
����һ��ȷ��ȡmg����ͭ������Ʒ�����250mL��Һ��
���������ȡ25.00mL��Һ����ƿ�У�����Һ�м�������KIҡ�ȣ���cmol/LNa2S2O3��Һ�ζ�����Һ��dz��ɫʱ������10mL10%KSCN�Լ������Ӽ��ε�����Һ��������cmol/LNa2S2O3��Һ�ζ����յ㣬������Na2S2O3��ҺV1mL��
����������25.00mL����ˮ�������ͭ��Һ���ظ������������Na2S2O3��ҺV2mL��
��֪��CuI������ˮ��������I2��2Cu2++4I-=2CuI��+I2��I2+2![]() =
=![]() +2I-��CuI(s)+SCN-(aq)
+2I-��CuI(s)+SCN-(aq)![]() CuSCN(s)+I-(aq)
CuSCN(s)+I-(aq)
��������Һʱ�õ��IJ��������У��ձ�����Ͳ����������__��
��ʵ���м���10mL10%KSCN�Լ���Ŀ����__��
�ۼ���ͭ����Ĵ���__(�б���ʽ���ɣ�Cu(HCOO)2��4H2O��Ħ������Ϊ226g/mol)��
���𰸡�����Ӵ������ʹ��Ӧ��ֽ��� �ṩ���Ի��������������ɼ�ʽ̼��ͭ�� �¶ȹ��ߣ��ᵼ�¼�ʽ̼��ͭ�ֽ���¶ȹ��ߣ�ˮ�ⷴӦ�̶ȼӴ����ɹ����Cu(OH)2�� ϴ�� �� ���������ᣬHCOONa��Һ��pH����7������δ��Ӧ��NaOH�����ʹ��Һ��pH����7 ��һ������ϡ�����ữ�μ����Ը��������Һ�ܹ���ɫ���𰸶�������������Һˮԡ�������������ɣ���������������������ͭ�������к�ɫ�������� 250mL����ƿ����ͷ�ι� ��CuIת��ΪCuSCN����ֹ������I2 ![]() ��100%
��100%
��������
��NaHCO3�����CuSO4��5H2O���������O���ټ�����ˮ�����ɵļ�ʽ̼��ͭ�������ˡ�ϴ�ӡ������ã�һ�������£�CO����NaOH���巢����Ӧ����HCOONa���ٽ�HCOONa�ữ�����ɵ�HCOOH����Ӧ���������ɵ�HCOONa����Һ�Լ��ԣ��Ҿ���ȩ�������ʣ����Cu(OH)2��CuCO3��HCOOH��һ������Ϸ�Ӧ�����Ƶü���ͭ���塣
(1)��NaHCO3�����CuSO4��5H2O���������O���ﵽ����Ӵ������ʹ��Ӧ��ֽ��е�Ŀ�ģ�
(2)��Ӧ2CuSO4+4NaHCO3�TCu(OH)2��CuCO3��+3CO2��+2Na2SO4+H2O���漰��HCO3-��ˮ������룬��ԭ���й�����NaHCO3���ṩ���Ի��������������ɼ�ʽ̼��ͭ����Cu(OH)2��CuCO3���ȶ��������ֽ⣬��Ӧ�¶ȵ���80�棬�ɷ�ֹ�¶ȹ��ߣ��ᵼ�¼�ʽ̼��ͭ�ֽ���¶ȹ��ߣ�ˮ�ⷴӦ�̶ȼӴ����ɹ����Cu(OH)2��
(3)������Һ�л��Cu(OH)2��CuCO3��������Ҫ�������ˡ�ϴ�ӡ������ã�
(4)�ټ��������ᣬHCOONa��Һ��pH����7������δ��Ӧ��NaOH�����ʹ��Һ��pH����7����ͨ���ⶨ��Ӧ������������Ƶ���ҺpH��7����֤����HCOONa���ɣ�
��HCOONa����ȩ�����л�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ��Ҳ�ܷ���������Ӧ����ɲ��ã�����һ������ϡ�����ữ�μ����Ը��������Һ�ܹ���ɫ��������������������Һˮԡ�������������ɣ�����������������������ͭ�������к�ɫ�������ɡ�
(5)������250mL��Һ���õ��IJ��������У��ձ�����Ͳ����������250mL����ƿ�ͽ�ͷ�ιܣ�
��CuI������ˮ��������I2�������10%KSCN�ɽ�CuIת��ΪCuSCN����ֹ������I2��
����֪��2Cu2++4I-==![]() +2I-��CuI(s)+SCN-(aq)
+2I-��CuI(s)+SCN-(aq)![]() CuSCN(s)+I-(aq)����2Cu(HCOO)2��4H2O~~~~2CuI~~~~~~~~I2~~~~~~~~2
CuSCN(s)+I-(aq)����2Cu(HCOO)2��4H2O~~~~2CuI~~~~~~~~I2~~~~~~~~2![]() ���ζ�����ʵ������Na2S2O3��Һ(V1-V2)mL�������ͭ����Ĵ���Ϊ
���ζ�����ʵ������Na2S2O3��Һ(V1-V2)mL�������ͭ����Ĵ���Ϊ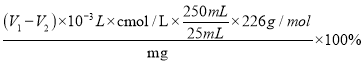 =
=![]() ��100%��
��100%��

 ���Ͱ�ͨ��ĩ���ϵ�д�
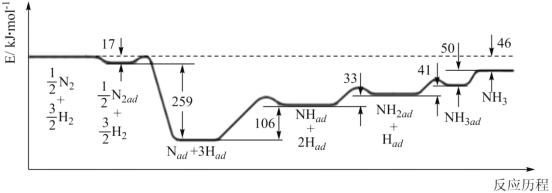
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ�����ڷ�ӦA(g)![]() 2B(g) H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ��
2B(g) H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ��

��1����������ͼ������˵������ȷ����___��
A��a��c����ķ�Ӧ���ʣ�c>a
B��b��c����A�����ת�������
C����״̬b��״̬a������ͨ�����ȵķ���
D����״̬b��״̬c������ͨ����ѹ�ķ���
��2����������Ӧ�ڶ����ܱ������н��У��ﵽƽ��״̬�ı�־��___��
A����λʱ��������nmolA��ͬʱ�ֽ�2nmolB B���������������������ٸı�
C�����������ܶȲ��ٷ����仯 D�����������������ٷ����仯
��3����������Ӧ��ƽ��ʱ��B�����ƽ��Ũ��Ϊ0.1mol��L-1��ͨ����С�����������ϵ��ѹǿ(�¶ȱ��ֲ���)�����´�ƽ���B�����ƽ��Ũ��___0.1mol��L-1(����ڡ�����С�ڡ����ڡ�)��
��4����100��ʱ����0.40mol��B�������2L�ܱ������У�ÿ��һ��ʱ��ͶԸ������ڵ����ʽ��з������õ���������ݣ�
ʱ��(s) | 0 | 20 | 40 | 60 | 80 |
n(B)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
n(A)/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
�ϱ���n3___n4(����ڡ�����С�ڡ����ڡ�)����ӦA(g)![]() 2B(g)��100��ʱ��ƽ�ⳣ��K��ֵΪ___�������¶Ⱥ�Ӧ2B(g)
2B(g)��100��ʱ��ƽ�ⳣ��K��ֵΪ___�������¶Ⱥ�Ӧ2B(g)![]() A(g)��ƽ�ⳣ��K��ֵ___(���������С�����䡱)��
A(g)��ƽ�ⳣ��K��ֵ___(���������С�����䡱)��
����Ŀ������ͼ��ʾװ�÷ֱ��������ʵ�飺��������Һ�������У���Ҫ����д���пո�

���� | �������� | �������� | ��ձ�ż�Ҫ�� |
ʵ��1 | �μӹ���̪��ˮ | Na2O2 | д���ῴ����������_____ |
ʵ��2 | NaOH��Һ | δ��ĥ�������� | д����������Ӧ�����ӷ���ʽ ��_____ |
ʵ��3 | ���� | NaAlO2��Һ | �������ɳ�����������ļ���ı仯ͼ�� ��_____ |
ʵ��4 | ϡ���� | Na2CO3��NaOH�Ļ����Һ | ��֪����������������ļ���ı仯ͼ�����£���ԭ��Һ�е�Na2CO3��NaOH�����ʵ���Ũ��֮��Ϊ ��_____
|
ʵ��5 | FeCl3������Ļ��Һ | ��������ͭ��п�������ֽ����Ļ���� | ����Ӧ������ʣ�࣬��Ӧ�����Һ��һ�����ڵ��������� ��_____ |
����Ŀ����γ�ȥ���ʡ��ᴿ���и����ʣ����ڱ�����ա�
�����ɷ� | �ᴿ�����Լ��Ļ�ѧʽ | ���ӷ���ʽ |
��1��FeCl2�����FeCl3���� | _______ | __________________ |
��2��FeCl3�����FeCl2���� | _______ | __________________ |
��3��FeSO4�����CuSO4���� | _______ | __________________ |