��Ŀ����
13���ϳɸ߷��Ӳ��ϵ����ƺ�ʹ�ü���ظı���������ճ���������ķ�չ����1�����й㷺��;�ľ۱�ϩ���ϵ�����Ϊ-CH2-CH��CH3��-��
��2���л������Ļ�ѧ���ƽоۼ���ϩ���������ṹ��ʽΪ
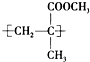 ��
����3����Ȼ���������ϩ�ľۺ���䵥���ϵͳ����Ϊ2-��-1��3-����ϩ��
��4��������£��ϳɷ�ȩ��֬�Ļ�ѧ����ʽΪ
 ��
����5�����д����Ͼ��ȹ��Է�ȩ��֬�������У���ѧ����������bc������ĸ���ţ���
A������ B�������������֬����
C������ȼ�� D�����л��ܼ������ܽ⣬������֬��
���� ��1���۱�ϩΪ��ϩͨ���Ӿ۷�Ӧ���ɵģ��ݴ��ж������ڣ�
��2������ϩ�����ͨ���Ӿ۷�Ӧ���ɾۼ���ϩ��������ݴ�д����ṹ��ʽ��
��3��������Ϊ1��3-����ϩ����2��C����1�������ݴ�д�������ƣ�
��4����ȩ��֬���ɱ��Ӻͼ�ȩ�ڴ������������۶��ɣ���Ӧ�����DZ����ǻ���λ�ϵ�������ԭ�ӱȽϻ��ã����ȩȩ���ϵ���ԭ�ӽ��Ϊˮ���ӣ����ಿ������������Ϊ�߷��ӻ�����--��ȩ��֬��
��5�����ݷϾɵĺϳɲ��Ͽ��������õ�֪ʶ��������
��� �⣺��1����ϩͨ���Ӿ۷�Ӧ���ɾ۱�ϩ����۱�ϩ������Ϊ��-CH2-CH��CH3��-��
�ʴ�Ϊ��-CH2-CH��CH3��-��
��2���ۼ���ϩ������Ǽ���ϩ�����ͨ���Ӿ۷�Ӧ���ɵģ���ṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3�������ϩ�Ľṹ��ʽΪ��CH2=C��CH3��-CH=CH2��������Ϊ��2-��-1��3-����ϩ��
�ʴ�Ϊ��2-��-1��3-����ϩ��
��4�������ǻ���λ�ϵ�������ԭ�ӱȽϻ��ã����ȩȩ���ϵ���ԭ�ӽ��Ϊˮ���ӣ����ಿ������������Ϊ�߷��ӻ�����--��ȩ��֬����Ӧ�ķ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��5���Ͼɺϳɲ��ϵ�����������Ҫ������;����ͨ���������ԣ��������ɶ������õIJ��Ϻ���Ʒ���������ѽ��ѧ��������ʹ��ֽ⣬�����Ʊ����ֻ���ԭ�ϣ����Ͼɵľۺ�����Ϊȼ�ϻ����������ܣ���������ܾò��ḯ�ã�����ɰ�ɫ��Ⱦ����ȩ���ϲ��������л��ܼ�������bc��ȷ��
��ѡbc��
���� ���⿼�����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ��漰�߷��ӻ����ﵥ�塢���ڡ��ṹ��ʽ����д���л���Ӧ����ʽ��д��֪ʶ����ȷ�����л���ṹ������Ϊ���ؼ�������������ѧ�����Ӧ�û���֪ʶ��������

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д� ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�| A�� | �����¼�������粒��壺NH4NO3$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+HNO3�� | |
| B�� | ����Ƭ�����ռ���Һ�У�2Al+2OH-+2H2O=2AlO2-+3H2�� | |
| C�� | ������������Һ�м����������SO32-+2H+=SO2��+H2O | |
| D�� | ��ҵ�ƴֹ裺C+SiO2$\frac{\underline{\;����\;}}{\;}$CO2��+Si |
| A�� | ��FeCl3��Һ����KSCN�����ӡ�NaOH���Ҵ���AgNO3��ˮ��Һ | |
| B�� | �ں����������ӵı��м�������Ũ��ˮ�����ˣ��Գ�ȥ���� | |
| C�� | �������е���ϩ�ɾ���ˮϴ��Һ���ȥ | |
| D�� | ���������е�������ȼ�NaOH��Һ��Ȼ���Һ��ȥ |
ʵ��С������0.8mol•L-1KI��Һ��0.1mol•L-1H2SO4��Һ��������Һ����̽���¶ȡ���ȶ�������Ӧ���ʵ�Ӱ�죬��������A-C����ʵ�飬����ʵ���������±���
| ��� | �¶�/�� | H2SO4���/mL | KI��Һ���/mL | H2O���/mL | ������Һ /mL | ������ɫʱ��/s |
| A | 39 | 10 | 5 | 5 | 1 | 5 |
| B | TB | 10 | 5 | 5 | 1 | û������ɫ |
| C | 5 | 10 | 5 | 5 | 1 | 39 |
| D | t |
��2��A-C����ʵ��ʱ����������5mLˮ����Ŀ����Ϊ���ں����о�������ضԷ�Ӧ����Ӱ��ʱ����KI�͵���Ũ�Ȳ��䣨������������Ʊ����������Ա�ʵ�顱��˼�ĸ��ֺ����𰸶��÷֣���
��3��B��ʵ���С�û������ɫ����ԭ�����¶ȣ�TB������75�棬������ⲻ��ɫ��
��4������ΪС�����D��ʵ�鷽�����ڱ���հ״���������Ƶ�5�����ݣ�������С�����̽��Ŀ�꣮
��5��������Ƶ�ʵ�����ݣ�����Ԥ��һ��tֵ����д������Ԥ�����Ӧ��̽��ʵ���������������10mLʱ��tԤ��ֵҪС��ͬ�¶Ա�ֵ������������10mLʱ��tԤ��ֵҪ����ͬ�¶Ա�ֵ�����ۣ����Խ��Ӧ����Խ�죮
| A�� | ��������ģ�ͣ� | B�� | ��CH3��3COH�����ƣ�2��2�����Ҵ� | ||
| C�� | ��ȩ�Ľṹʽ��CH3CHO | D�� | �ǻ��ĵ���ʽ��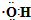 |
| A�� | �飨As��λ�����ڱ���4���ڵڢ�A�� | B�� | ��̬�⻯���ȶ��ԣ�AsH3��PH3��NH3 | ||
| C�� | ���������ԣ�H3AsO4��HNO3��H3PO4 | D�� | �⻯��е㣺AsH3��PH3��NH3 |
| A�� | ��ϡ��ˮ��ͨ�����CO2��NH3•H2O+CO2=NH4++HCO3- | |
| B�� | SO2+H2O+Ca2++2ClO-=CaSO3��+2HClO | |
| C�� | ��Ba��OH��2��Һ�м���������NaHSO3��Һ2HSO3-+Ba2++2OH-��BaSO3��+SO32-+2H2O | |
| D�� | ����������Һ������ʵ�����ϡ�����ϣ�Ca2++OH-+H++SO42-=CaSO4��+H2O |
 C70��������������״�Ķ����壬�ýṹ�Ľ����������¿���
C70��������������״�Ķ����壬�ýṹ�Ľ����������¿��� ij�о�ѧϰС������ͼװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�ѧϰС������ͼװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���