ЬтФПФкШн
ЁОЬтФПЁПСђЛЏЬМгжГЦбѕСђЛЏЬМ(ЛЏбЇЪНЮЊCOS)ЃЌЪЧХЉвЉЁЂвНвЉКЭЦфЫќгаЛњКЯГЩЕФживЊдСЯЁЃCOSЕФКЯГЩЗНЗЈжЎвЛЪЧдкЮоШмМСЕФЬѕМўЯТгУCOгыСђеєЦјЗДгІжЦЕУЃЌИУЗЈСїГЬМђЕЅЁЂЪеаЇИпЃЌЕЋКЌгаCO2ЁЂSO2ЕШдгжЪЁЃ
ЃЈ1ЃЉCOSЕФЕчзгЪНЮЊ_________ЁЃ
ЃЈ2ЃЉвбжЊCO2ДпЛЏМгЧтКЯГЩввДМЕФЗДгІдРэЪЧ: 2CO2(g)+6H2(g)![]() C2H5OH+3H2O(g) ЁїH=-173.6kJ/mol
C2H5OH+3H2O(g) ЁїH=-173.6kJ/mol
ЯТЭМЪЧВЛЭЌЦ№ЪМЭЖСЯЪБЃЌCO2ЕФЦНКтзЊЛЏТЪЫцЮТЖШБфЛЏЕФЙиЯЕ,ЭМжаm=![]() ЃЌЮЊЦ№ЪМЪБЕФЭЖСЯБШЃЌдђm1ЁЂm2ЁЂm3ДгДѓЕНаЁЕФЫГађЮЊ_______ЃЌРэгЩЪЧ____________ЁЃ
ЃЌЮЊЦ№ЪМЪБЕФЭЖСЯБШЃЌдђm1ЁЂm2ЁЂm3ДгДѓЕНаЁЕФЫГађЮЊ_______ЃЌРэгЩЪЧ____________ЁЃ

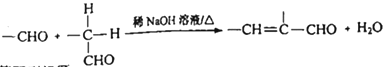
ЃЈ3ЃЉЬьШЛЦјВПЗжбѕЛЏжЦШЁЕФЛЏЙЄдСЯЦјжаЃЌГЃКЌгаCOSЁЃФПЧАCOSЫЎНтЪЧЭбГ§COSЕФГЃМћЗНЗЈЃЌМДCOSдкДпЛЏМСзїгУЯТгыЫЎеєЦјЗДгІЩњГЩСђЛЏЧтЃЌЩњГЩЕФСђЛЏЧтПЩгУбѕЛЏаПЕШЭбСђМСЭбГ§ЁЃ
ЂйCOSЫЎНтЕФЛЏбЇЗНГЬЪНЮЊ_________________ЁЃ
ЂкГЃЮТЯТЃЌЪЕбщВтЕУЭбСђ(ЭбçѐˏЧт)ЗДгІЙ§ГЬжаЃЌУПЯћКФ4.05gZnOЃЌЗХГі3.83kJШШСПЁЃИУЭбСђЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ_________________ЁЃ
ЂлНќФъРДЃЌЕчЛЏбЇМфНгбѕЛЏЗЈДІРэСђЛЏЧтЕФММЪѕЕУЕННЯПьЗЂеЙЁЃИУЗНЗЈЪЧРћгУFe3+дкЫсадЬѕМўЯТгыH2SЗДгІЩњГЩСђЕЅжЪЃЌЗДгІКѓЕФШмвКдйгУЕчНтЕФЗНЗЈЁАдйЩњЁБЃЌЪЕЯжбЛЗРћгУЁЃЕчНтЗЈЪЙFe3+ЁАдйЩњЁБЕФРызгЗНГЬЪНЮЊ___________ЃЌИУЗДгІЕФбєМЋВФСЯЪЧ______ЁЃ
ЂмГЃЮТЯТЃЌHClКЭCuCl2ЕФЛьКЯШмвКжаЃЌc(H+)=0.30mol/LЃЌc(Cu2+)=0.10mol/LЃЌЭљИУШмвКжаЭЈШыH2SжСБЅКЭ(H2SЕФНќЫЦХЈЖШЮЊ0.10mol/L)ЃЌ_____(ЬюЁАФмЁБЛђЁАВЛФмЁБ)ГіЯжГСЕэ,гУБивЊЕФМЦЫуЙ§ГЬКЭЮФзжЫЕУїРэгЩЁЃ
(вбжЊKa1(H2S)=1.3ЁС10-7ЃЌKa2(H2S)=7.0ЁС10-5ЃЌKsp(CuS)=1.4ЁС10-36)
ЁОД№АИЁП ![]() m1>m2>m3 ЮТЖШЯрЭЌЪБЃЌЭЖСЯБШmдіДѓЃЌдіМгH2ЕФСПЃЌCO2зЊЛЏТЪдіДѓ COS+H2O
m1>m2>m3 ЮТЖШЯрЭЌЪБЃЌЭЖСЯБШmдіДѓЃЌдіМгH2ЕФСПЃЌCO2зЊЛЏТЪдіДѓ COS+H2O![]() CO2+H2S ZnO(s)+H2S(g)=ZnS(s)+H2O(l) ЁїH=-76.6kJ/mol Fe2++2H+
CO2+H2S ZnO(s)+H2S(g)=ZnS(s)+H2O(l) ЁїH=-76.6kJ/mol Fe2++2H+![]() 2Fe3++H2Ёќ ЬМАєЁЂВЌАєЕШЖшадЕчМЋВФСЯ Фм
2Fe3++H2Ёќ ЬМАєЁЂВЌАєЕШЖшадЕчМЋВФСЯ Фм
ЁОНтЮіЁПЃЈ1ЃЉCOSЕФЕчзгЪНЮЊ![]() ЃЛЃЈ2ЃЉm=
ЃЛЃЈ2ЃЉm=![]() дНДѓЃЌдНгаРћгкЖўбѕЛЏЬМЕФзЊЛЏЃЌЖўбѕЛЏЬМЕФзЊЛЏТЪдНДѓЃЌЙЪm1>m2>m3ЃЌРэгЩЪЧЮТЖШЯрЭЌЪБЃЌЭЖСЯБШmдіДѓЃЌдіМгH2ЕФСПЃЌCO2зЊЛЏТЪдіДѓЃЛЃЈ3ЃЉЂйCOSЫЎНтЩњГЩЖўбѕЛЏЬМКЭЧтСђЫсЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊCOS+H2O
дНДѓЃЌдНгаРћгкЖўбѕЛЏЬМЕФзЊЛЏЃЌЖўбѕЛЏЬМЕФзЊЛЏТЪдНДѓЃЌЙЪm1>m2>m3ЃЌРэгЩЪЧЮТЖШЯрЭЌЪБЃЌЭЖСЯБШmдіДѓЃЌдіМгH2ЕФСПЃЌCO2зЊЛЏТЪдіДѓЃЛЃЈ3ЃЉЂйCOSЫЎНтЩњГЩЖўбѕЛЏЬМКЭЧтСђЫсЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊCOS+H2O![]() CO2+H2SЃЛЂкГЃЮТЯТЃЌЪЕбщВтЕУЭбСђ(ЭбçѐˏЧт)ЗДгІЙ§ГЬжаЃЌУПЯћКФ4.05gМД0.05molZnOЃЌЗХГі3.83kJШШСПЃЌЙЪШєЯћКФ1molZnOдђЗХГі76.6kJШШСПЁЃИУЭбСђЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊZnO(s)+H2S(g)=ZnS(s)+H2O(l) ЁїH=-76.6kJ/molЃЛЂлЕчНтЗЈЪЙFe3+ЁАдйЩњЁБЪЧдкЫсадЬѕМўЯТFe2+ЪЇЕчзгВњЩњFe3+ЃЌЭЌЪБЩњГЩЧтЦјЃЌЗДгІЕФРызгЗНГЬЪНЮЊFe2++2H+
CO2+H2SЃЛЂкГЃЮТЯТЃЌЪЕбщВтЕУЭбСђ(ЭбçѐˏЧт)ЗДгІЙ§ГЬжаЃЌУПЯћКФ4.05gМД0.05molZnOЃЌЗХГі3.83kJШШСПЃЌЙЪШєЯћКФ1molZnOдђЗХГі76.6kJШШСПЁЃИУЭбСђЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊZnO(s)+H2S(g)=ZnS(s)+H2O(l) ЁїH=-76.6kJ/molЃЛЂлЕчНтЗЈЪЙFe3+ЁАдйЩњЁБЪЧдкЫсадЬѕМўЯТFe2+ЪЇЕчзгВњЩњFe3+ЃЌЭЌЪБЩњГЩЧтЦјЃЌЗДгІЕФРызгЗНГЬЪНЮЊFe2++2H+![]() 2Fe3++H2ЁќЃЌИУЗДгІЕФбєМЋВФСЯЪЧЬМАєЁЂВЌАєЕШЖшадЕчМЋВФСЯЃЛЂмc(H+)=0.30mol/LЃЌc(Cu2+)=0.10mol/LЃЌc(H2S)
2Fe3++H2ЁќЃЌИУЗДгІЕФбєМЋВФСЯЪЧЬМАєЁЂВЌАєЕШЖшадЕчМЋВФСЯЃЛЂмc(H+)=0.30mol/LЃЌc(Cu2+)=0.10mol/LЃЌc(H2S)![]() 0.10mol/LЃЌKa1(H2S)=
0.10mol/LЃЌKa1(H2S)=![]() =
=![]() =1.3ЁС10-7ЃЌ
=1.3ЁС10-7ЃЌ![]() ЃЌKa2(H2S)=
ЃЌKa2(H2S)=![]() =
=![]() =7.0ЁС10-5ЃЌ
=7.0ЁС10-5ЃЌ![]() ЃЌQ sp(CuS)=
ЃЌQ sp(CuS)=![]()
![]() >Ksp(CuS)=1.4ЁС10-36)ЃЌЙЪФмГіЯжГСЕэЁЃ
>Ksp(CuS)=1.4ЁС10-36)ЃЌЙЪФмГіЯжГСЕэЁЃ