��Ŀ����
3��3-��़��ᣨ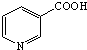 ���׳����ᣬ����ˮ������Ѫ��������ҩ����������գ�
���׳����ᣬ����ˮ������Ѫ��������ҩ����������գ���1�����������������ж���ȷ����c����ѡ���ţ�
a��ֻ������ b��ֻ�м��� c�������������м���
��2��д������ĺ�������ͬ���칹��Ľṹ��ʽ��ͬʱд���ϳɸ�����������Լ���
�ṹ��ʽ
 ���Լ�Ũ���ᡢŨ���ᣮ
���Լ�Ũ���ᡢŨ���ᣮ��3��ʵ���Һϳ�����ķ�����ͼ��

д��A��B�Ļ�ѧʽ��AKMnO4 BH2SO4
�����ᾭ�����ᴿ������ɻ�ô��������ᣮA��Ҫ�ֶ�μ��뵽��Ӧ��ϵ�У�˵��ԭ�������ų���������Ӧ��Ҫ�����¶���70�森
���� ��1��3-��़��Ậ���Ȼ��������������ʣ�Nԭ���йµ��Ӷԣ����������ӽ�ϣ����������ԣ�
��2������ĺ�������ͬ���칹��Ϊ������������Ũ���ᡢŨ�����ڼ���������������������
��3����3-��़���Ľṹ��ʽ����֪3-�����Ϊ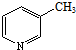 ���ӻ��뱽���������ƣ��ڼ����������ø��������Һ�����õ�
���ӻ��뱽���������ƣ��ڼ����������ø��������Һ�����õ�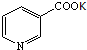 ����Ӧ��Ҫ�����¶���70�棬Ӧ�����������Һ�ֶ�μ��뵽��Ӧ��ϵ�У��������˷��룬��Һ�������ữ�õ�
����Ӧ��Ҫ�����¶���70�棬Ӧ�����������Һ�ֶ�μ��뵽��Ӧ��ϵ�У��������˷��룬��Һ�������ữ�õ� ��������������ˮ�����˿ɵ����ᣮ
��������������ˮ�����˿ɵ����ᣮ
��� �⣺��1��3-��़��Ậ���Ȼ��������������ʣ�Nԭ���йµ��Ӷԣ����������ӽ�ϣ����������ԣ���ѡ��C��
��2������ĺ�������ͬ���칹��Ϊ���������ṹ��ʽΪ ������Ũ���ᡢŨ�����ڼ����������������������ʴ�Ϊ��
������Ũ���ᡢŨ�����ڼ����������������������ʴ�Ϊ�� ��Ũ���ᡢŨ���
��Ũ���ᡢŨ���
��3����3-��़���Ľṹ��ʽ����֪3-�����Ϊ ���ӻ��뱽���������ƣ��ڼ����������ø��������Һ�����õ�
���ӻ��뱽���������ƣ��ڼ����������ø��������Һ�����õ� ������3-����������ų���������Ӧ��Ҫ�����¶���70�棬Ӧ�����������Һ�ֶ�μ��뵽��Ӧ��ϵ�У��������˷��룬��Һ�������ữ�õ�
������3-����������ų���������Ӧ��Ҫ�����¶���70�棬Ӧ�����������Һ�ֶ�μ��뵽��Ӧ��ϵ�У��������˷��룬��Һ�������ữ�õ� ��������������ˮ�����˿ɵ����ᣬ�����ᾭ�����ᴿ������ɻ�ô��������ᣬ
��������������ˮ�����˿ɵ����ᣬ�����ᾭ�����ᴿ������ɻ�ô��������ᣬ
�ʴ�Ϊ��KMnO4��H2SO4������3-����������ų���������Ӧ��Ҫ�����¶���70�森
���� ���⿼���л���ĺϳɡ��л���Ľṹ�����ʣ���Ŀ��������ѧ�������漰���ѶȽϴ�

| A�� | ˮ�ĵ��뷽��ʽ��H2O=H++OH- | |
| B�� | pH=7����Һһ����������Һ | |
| C�� | �����¶ȣ�ˮ�ĵ���̶����� | |
| D�� | ��ϡ�����ˮϡ��ʱ��c��H+����С��c��OH-��Ҳ��С |
| A�� | ������ | B�� | ������ | C�� | ������ | D�� | ������ |
| A�� | Ũ���� | B�� | ��ʯ�� | C�� | �������� | D�� | ����̿ |
 ��
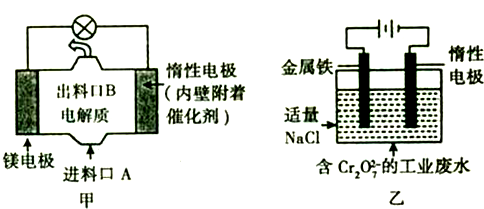
��
 ��
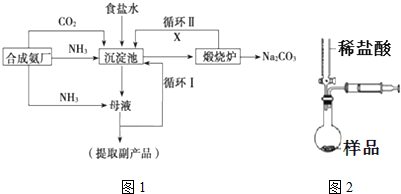
��
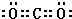 ���������з����Ļ�ѧ��Ӧ����ʽΪ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl��
���������з����Ļ�ѧ��Ӧ����ʽΪ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl��