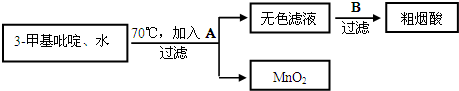
��Ŀ����
13���ҹ���ѧ�Һ�°�Ľ�����Ĵ����������գ��������̿ɼ�Ҫ��ʾ��ͼ1��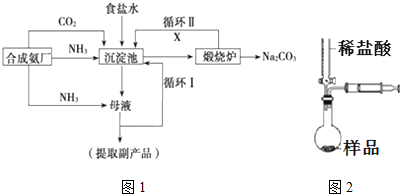
��1��д������������ѭ��������X�ĵ���ʽ
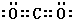 ���������з����Ļ�ѧ��Ӧ����ʽΪ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl��
���������з����Ļ�ѧ��Ӧ����ʽΪ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl����2����ĸҺ��ͨ�백��������ϸСʳ�ο�������ȴ��������Ʒ����ƷΪNH4Cl��
��3������������ˢ�ѭ����Ŀ�������ԭ���Ȼ��Ƶ������ʣ�
��4�������ӷ���ʽ��ʾNa2CO3��Һ�ʼ��Ե�ԭ��CO32-+H2O?HCO3-+OH-��
��5����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����BC������ţ���
A��c��Na+��=2c��CO32-�� B��c��Na+����c��CO32-����c��HCO3-��
C��c��OH-����c��HCO3-����c��H+�� D��c��OH-��-c��H+��=c��HCO3-��+c��H2CO3��
��6��ijͬѧ��ƵIJⶨ��Ʒ��̼���ƺ����ķ�����ͼ2����ζ��ܵ���ʼ����ΪV1 mL���յ����ΪV2 mL��ע�����ⶨ�ų�������ΪV3 mL����ת���ɱ�״�������Ƶ���Ʒ����Ϊm g����ԭ��Ʒ��̼���Ƶ����������ı���ʽΪ$106��\frac{{V}_{3}-��{V}_{2}-{V}_{1}��}{22400m}��100%$���ú�V1��V2��V3��m�Ĵ���ʽ��ʾ����Ӧǰ����Һ�ܶȵı仯���Բ��ƣ���
���� ��1��������̼�Ƿ�Ӧ��ԭ��ͬʱҲ�Ƿ�Ӧ�ĸ��������ѭ�����ã��������ͼ��֪����������ѭ��������X�Ƕ�����̼��������̼�д�������̼��˫���������Ļ�ѧ��ӦΪ����ʳ��ˮ��ͨ�백���Ͷ�����̼����̼�����ƾ��壻
��2�����ݷ�Ӧ����ʽȷ��ĸҺ�е����ʣ����ݰ�������ˮ�����ɰ�ˮ����ˮ�����笠�������笠���Ũ���������Ȼ�淋�������������
��3�����ԭ�ϵ������ʣ�������ѭ��ʹ�õķ�����
��4��̼����Ϊǿ�������Σ�̼�������ˮ�����Һ�ʼ��ԣ�
��5��Na2CO3����Һ�е���ˮ�⣺Na2CO3 =2Na++CO32-��CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-��H2O?H++OH-��������غ㡢����غ���
��6���ҳ�������̼�����������̼�غ�����̼���Ƶ����ʵ���������m=nM����̼���Ƶ��������������̼���Ƶ�����������
��� �⣺��1�������������Ƽ�ж�����̼�Ƿ�Ӧ��ԭ��ͬʱҲ�Ƿ�Ӧ�ĸ��������ѭ�����ã���������������ѭ��������X�Ƕ�����̼��������̼Ϊֱ���ͽṹ�������д�������̼��˫����������̼�ĵ���ʽΪ��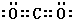 ���������з����Ļ�ѧ��ӦΪ����ʳ��ˮ��ͨ�백���Ͷ�����̼����̼�����ƾ��壬��Ӧ����ʽΪNH3+H2O+CO2+NaCl=NH4Cl+NaHCO3����
���������з����Ļ�ѧ��ӦΪ����ʳ��ˮ��ͨ�백���Ͷ�����̼����̼�����ƾ��壬��Ӧ����ʽΪNH3+H2O+CO2+NaCl=NH4Cl+NaHCO3����
�ʴ�Ϊ��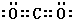 ��NH3+H2O+CO2+NaCl=NH4Cl+NaHCO3����
��NH3+H2O+CO2+NaCl=NH4Cl+NaHCO3����
��2������NH3+H2O+CO2+NaCl�TNH4Cl+NaHCO3��������ͼ֪��ĸҺ������Ϊ�Ȼ�泥���ĸҺ��ͨ��������ϸСʳ�ο�������ȴ��������Ʒ��ͨ��İ�����ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ������笠����ӣ�笠�����Ũ�����������������Ȼ�泥�
�ʴ�Ϊ��NH4Cl��
��3��ѭ�����ǽ�δ��Ӧ���Ȼ��Ʒ��س������У����ԭ���Ȼ��Ƶ������ʣ�
�ʴ�Ϊ�����ԭ���Ȼ��Ƶ������ʣ�
��4��̼����Ϊǿ�������Σ������Ӳ�ˮ�⡢̼�������ˮ�����Һ�ʼ��ԣ�ˮ�����ӷ���ʽΪ��CO32-+H2O?HCO3-+OH-��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
��5��A��Na2CO3 =2Na++CO32-����̼�������ˮ�⣬������Ũ�ȴ�С��ϵΪ��c��Na+����2c��CO32-������A����
B�����������ˮ��̶Ⱥ�С������Һ�д�������ˮ�⣺CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-���Ե�һ��ˮ��Ϊ����������Ũ�ȴ�С��ϵΪ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+������B��ȷ��
C��̼�������ˮ������̼��������Ӻ����������ӣ���Һ�Լ��ԣ�c��OH-����c��H+������Һ�д�������ˮ�⣺CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-���Ե�һ��ˮ��Ϊ��������c��OH-����c��HCO3-����c��H+������C��ȷ��
D��Na2CO3�������������Ӻ�������������Һ�����е���ԭ�Ӻ���������������ˮ����ȣ�����CO32-ˮ���Ĵ�����ʽΪCO32-��HCO3-��H2CO3�����У�c��H+��+c��HCO3-��+2c��H2CO3��=c��OH-��������c��OH-��-c��H+��=c��HCO3-��+2c��H2CO3������D����
�ʴ�Ϊ��BC��
��6�������ų��Ŀ���������ǣ�V2-V1��mL�������Ķ�����̼�������V3-��V2-V1��������̼ԭ���غ㣬̼���Ƶ����ʵ����ǣ�$\frac{V_{3}-��V_{2}-V_{1}��}{22400}$mol��ԭ��Ʒ��̼���Ƶ���������Ϊ��$106��\frac{{V}_{3}-��{V}_{2}-{V}_{1}��}{22400m}��100%$��
�ʴ�Ϊ��106��$\frac{V3-��V2-V1��}{22400m}$��100%��
���� ���⿼���˺����Ƽ��Ӧԭ����̼���ƺ����IJⶨ���漰�˻�ѧ����ʽ����д�����������ļ����֪ʶ�����ݽ϶࣬ע�⣨5�����������غ㡢����غ���Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ���쳣������ɫ�����Ϳ�� | |
| B�� | ������������ˮ�� | |
| C�� | ��������������Ư�� | |
| D�� | �����Ƴ����������������������Դ |
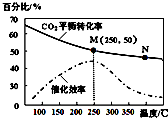
| A�� | ������ϩ�����ʣ�v��M���п���С��v��N�� | |
| B�� | ƽ�ⳣ����KM��KN | |
| C�� | ���¶ȸ���250�棬�����¶ȣ�ƽ�����淴Ӧ�����ƶ����Ӷ�ʹ�����Ĵ�Ч�ʽ��� | |
| D�� | ��Ͷ�ϱ�n��H2����n��CO2��=3��1����ͼ��M��ʱ����ϩ���������Ϊ7.7% |
| A�� | �����£�CH3COONa��CaCl2�����Һ��c��Na+��+c��Ca2+��=c��CH3COO-��+c��CH3COOH��+2c��Cl-�� | |
| B�� | ��ͬ�¶��£�0.6 mol•L-1��ˮ��0.3 mol•L-1��ˮ��c��OH-��֮����2��1 | |
| C�� | ��0.1 mol•L-1NaNO3��Һ�еμ�����ʹ��Һ��pH=5����ʱ���Һ�е�c��Na+��=c��NO3-�� | |
| D�� | �����£�0.1 mol•L-1NH4Cl��Һ��0.1 mol•L-1��ˮ�������ϣ�pH��7������c��NH3•H2O����c��NH4+����c��Cl-����c��OH-�� |
| A�� | NH4+��H+��NO3-��HCO3- | B�� | K+��Al3+��SO42-��NH3•H2O | ||
| C�� | Na+��K+��SO32-��Cl2 | D�� | Na+��Mg2-��SO42-��H+ |
| A�� | ���ԣ�HClO4��HBrO4��HIO4 ���ԣ�NaOH��KOH��RbOH | |
| B�� | ԭ�Ӱ뾶��Na��O��F ���Ӱ뾶����Na+��O2-��F- | |
| C�� | �ȶ��ԣ���HF��H2O��H2S ��ԭ�ԣ�HCl��H2S��PH3 | |
| D�� | ��ԭ�ԣ�Na��Mg��Al �����ԣ�P��S��Cl2 |
| A�� | ʯ��ʯ�ڸ����µķֽⷴӦ | B�� | �����ƺ�ˮ�ķ�Ӧ | ||
| C�� | ����������������Һ�ķ�Ӧ | D�� | ľ̿��������ȼ�� |
| A�� | ��ͼ�����顢��ϩ����Ȳ�Ľṹ�ֱ��ʾΪ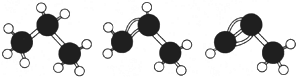 | |
| B�� | ��ͬ���ʵ���������������ȫȼ�գ����ɵ������ڱ�״̬�£������3��2��1 | |
| C�� | ���顢��ϩ����Ȳ�������ʵ��۷е������ߣ���ͬ�������ܶ������� | |
| D�� | ��Ȳ��̼ԭ��ռ�ṹ���������� |
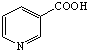 ���׳����ᣬ����ˮ������Ѫ��������ҩ����������գ�
���׳����ᣬ����ˮ������Ѫ��������ҩ����������գ�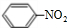 ���Լ�Ũ���ᡢŨ���ᣮ
���Լ�Ũ���ᡢŨ���ᣮ