��Ŀ����
(15��)
�Ķ��������ϣ��ݴ��������Ҫ��
��ҵ�����г�����������NaCl��NaOH��ijУ����С���ͬѧΪ�ⶨ������Ʒ��
Na2CO3������������ѡ�������ʵ�װ�ý������ӣ�������Ʒ����������ƣ��ɹ������
�ⶨʵ�顣
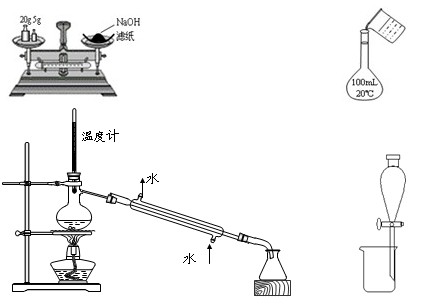
�ŻС�����λͬѧ��ʵ��װ�õ���ƽ��������۷��ԣ��������£�
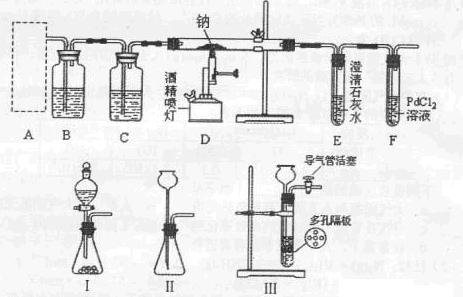
�ף���װ��A��E���Ӽ��ɡ���ΪNa2CO3��ϡ���ᷴӦ���ɵ�CO2��E�еļ�ʯ�����գ��ֱ������Ӧǰ�ͷ�Ӧ��E�����������Ϳ����Na2CO3������������
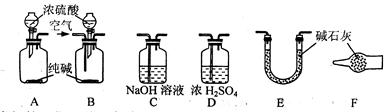
�ң���Ӧ��A��E֮������װ�� (�����)����Ŀ���� ��
����Ӧ����Bװ��ȡ��Aװ�á���Bװ�õĵ��ܿڴ����������������Ŀ���� ��
������ֱ����Bװ���й�������ᵼ��ʵ��ⶨ��� (�ƫ�ߡ�����ƫ�͡���
����Ӱ�족)����ˣ�Ӧ������Ŀ�����ͨ��װ�� (�����)��
�죺Eװ�ú�Ӧ����װ��F���������� ��
��ѧ������С��ⶨ���������£�����ù�ҵ������Na2CO3����������Ϊ ��
�Ǽ���ʵ���Ҳⶨ������Ʒ��Na2CO3��������������һ�ַ���(��ʵ��������ʵ�鲽�衢ʵ������) ��
�Ķ��������ϣ��ݴ��������Ҫ��
��ҵ�����г�����������NaCl��NaOH��ijУ����С���ͬѧΪ�ⶨ������Ʒ��
Na2CO3������������ѡ�������ʵ�װ�ý������ӣ�������Ʒ����������ƣ��ɹ������
�ⶨʵ�顣
 |
�ŻС�����λͬѧ��ʵ��װ�õ���ƽ��������۷��ԣ��������£�
�ף���װ��A��E���Ӽ��ɡ���ΪNa2CO3��ϡ���ᷴӦ���ɵ�CO2��E�еļ�ʯ�����գ��ֱ������Ӧǰ�ͷ�Ӧ��E�����������Ϳ����Na2CO3������������
�ң���Ӧ��A��E֮������װ�� (�����)����Ŀ���� ��
����Ӧ����Bװ��ȡ��Aװ�á���Bװ�õĵ��ܿڴ����������������Ŀ���� ��
������ֱ����Bװ���й�������ᵼ��ʵ��ⶨ��� (�ƫ�ߡ�����ƫ�͡���
����Ӱ�족)����ˣ�Ӧ������Ŀ�����ͨ��װ�� (�����)��
�죺Eװ�ú�Ӧ����װ��F���������� ��
��ѧ������С��ⶨ���������£�����ù�ҵ������Na2CO3����������Ϊ ��
| ��ҵ������Ʒ���� | ��ӦǰE�������� | ��Ӧ��E�������� |
| 6.4g | 51.9g | 54.1g |
(1)D(1��) ��ȥˮ(2��) ʹ������̼����ų���������ȫ����(2��)
ƫ��(1��) C(1��) ��ֹ�����еĶ�����̼��ˮ��������Eװ�ã�Ӱ��ⶨ���(3��)
(2)82.8% (3��)
(3)��������ƽ��ȡһ����������Ʒ(m1)���ձ��У������������ܽ⣬����������BaCl2��Һ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ���ɣ���ȡ����������(m2)����m1��m2�����Na2CO3����������(3��)
ƫ��(1��) C(1��) ��ֹ�����еĶ�����̼��ˮ��������Eװ�ã�Ӱ��ⶨ���(3��)
(2)82.8% (3��)
(3)��������ƽ��ȡһ����������Ʒ(m1)���ձ��У������������ܽ⣬����������BaCl2��Һ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ���ɣ���ȡ����������(m2)����m1��m2�����Na2CO3����������(3��)
�漰����������װ��ʵ�鷽�������ۣ������������ļ����ʵ�鷽������ƣ�������2005��ȫ����Iʵ�����ƣ����д��£�ֵ�÷���һ�£����������ͼ

��ϰ��ϵ�д�
�����Ŀ

 8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ���
8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ���
 .Na202 b��Na2C03 c��NaHCO3 d.Na2C03��C
.Na202 b��Na2C03 c��NaHCO3 d.Na2C03��C ��ȴ
��ȴ
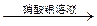 ���ְ�ɫ����
���ְ�ɫ����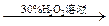
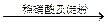 ��Һ����ɫ
��Һ����ɫ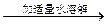
 �ؽᾧ
�ؽᾧ