��Ŀ����
��15�֣�
����������ѧ��ѧʵ���г��õ�����������ʵ������У�һ�㲻��Ҫ�ò���������
����д��ţ�
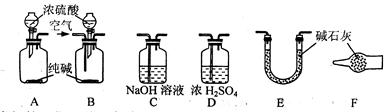
����pH��ֽ�ⶨNa2CO3��Һ��pH�� ������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ�� �۽������Ȼ���������Һ�����ˮ���Ʊ������������壻 ��̽��Ba(OH)2 8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ���
8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ���
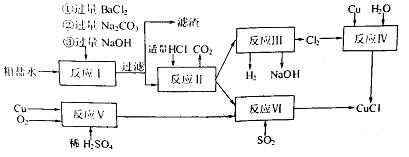
��Ϊ�ⶨij��������Na2O��Na2O2��Ʒ�Ĵ��ȣ�ijС��ͬѧ�ֱ���������·�����
������һ��ȷ������Ʒmg����ˮ��ַ�Ӧ����Һ�����ϡ��ΪVmL������ȡ��V1mL��Һ��װ����ƿ������֪Ũ�ȵ�������еζ�����ȷ����Һ��Ũ�ȣ��ټ������Ʒ��Na2O2�ĺ�����
��1���˷����У�����к͵ζ�ʱӦѡ��ָʾ���� ��
����������ȷ������Ʒmg������Ʒ�������̼��ַ�Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����
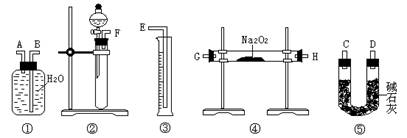
��2���÷�����ʵ������У�����������˳���� ���������·���ţ������еĽ�����Ϊ ���A����B����
��3��װ�âݵ������� ��
��4���ڿɹ�ѡ�õķ�Ӧ��ֻ��CaCO3����,6mol/L���������ˮʱ�������һ����IJⶨNa2O2���ȵ�ʵ�鷽�� ��
����������ѧ��ѧʵ���г��õ�����������ʵ������У�һ�㲻��Ҫ�ò���������
����д��ţ�
����pH��ֽ�ⶨNa2CO3��Һ��pH�� ������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ�� �۽������Ȼ���������Һ�����ˮ���Ʊ������������壻 ��̽��Ba(OH)2
 8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ���
8H2O�����NH4Cl���巴Ӧ�����е������仯�� �������������ַе���ϴ��Һ�壻 ���˷��뻥�����ܵĹ����Һ�壻 ������֪Ũ�ȵ�����ζ�����Ũ�ȵ�NaOH��Һ������к͵ζ����̣� ��ϡ��ŨH2SO4�Ĺ�����Ϊ�ⶨij��������Na2O��Na2O2��Ʒ�Ĵ��ȣ�ijС��ͬѧ�ֱ���������·�����
������һ��ȷ������Ʒmg����ˮ��ַ�Ӧ����Һ�����ϡ��ΪVmL������ȡ��V1mL��Һ��װ����ƿ������֪Ũ�ȵ�������еζ�����ȷ����Һ��Ũ�ȣ��ټ������Ʒ��Na2O2�ĺ�����
��1���˷����У�����к͵ζ�ʱӦѡ��ָʾ���� ��
����������ȷ������Ʒmg������Ʒ�������̼��ַ�Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����

��2���÷�����ʵ������У�����������˳���� ���������·���ţ������еĽ�����Ϊ ���A����B����
��3��װ�âݵ������� ��
��4���ڿɹ�ѡ�õķ�Ӧ��ֻ��CaCO3����,6mol/L���������ˮʱ�������һ����IJⶨNa2O2���ȵ�ʵ�鷽�� ��
��ۢݢߣ�3�֣���ŷ�̪�����ȣ���1�֣�
�Ƣڢܢݢ٢ۣ�3�֣�A��1�֣�
�dz�ȥO2�л��е�CO2�����壨3�֣�
�Ƚ���Ʒ�����ˮ��Ӧ�������O2���������4�֣�
�Ƣڢܢݢ٢ۣ�3�֣�A��1�֣�
�dz�ȥO2�л��е�CO2�����壨3�֣�
�Ƚ���Ʒ�����ˮ��Ӧ�������O2���������4�֣�
��

��ϰ��ϵ�д�
 ��������������������ϵ�д�
��������������������ϵ�д�
�����Ŀ