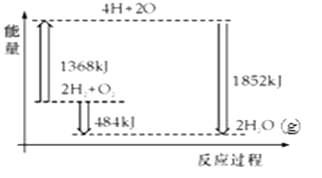
��Ŀ����
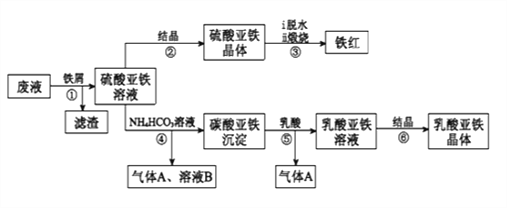
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4]����������Ͳ�Ѫ�������������������������£�
��֪��TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42-��TiOSO4ˮ���TiO2xH2O����Ϊ���淴Ӧ������ṹ��ʽΪCH3CH(OH)COOH��
��ش�
��1���������з�������������Һ�������IJ�����________________________��
��2��������м��Ŀ��һ�ǻ�ԭ����Fe2(SO4)3������ʹ����TiOSO4ת��ΪTiO2xH2O��������ƽ���ƶ���ԭ�����͵õ�������ԭ��___________________________��
��3�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ_________________��
��4�������ӷ���ʽ���Ͳ������м������ܵõ�����������ԭ��_________________��
��5�������������ӷ���ʽ��_________________________________________��
��6��Ϊ�ⶨ�����������þ�����FeSO4��7H2O������������ȡ������Ʒa g������ϡ�������100.00 mL��Һ��ȡ��20.00 mL��Һ����KMnO4��Һ�ζ���������KMnO4����Ӧ����������0.1000 molL-1 KMnO4��Һ20.00 mL�����þ�����FeSO4��7H2O����������Ϊ______________����a��ʾ����
���𰸡� ���� TiOSO4+(x+1)H2O![]() TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O
TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O![]() TiO2 xH2O��+2H+����м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O���� 1: 4 FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� Fe2++2HCO3-==FeCO3��+H2O+CO2�� 13.9/a
TiO2 xH2O��+2H+����м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O���� 1: 4 FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� Fe2++2HCO3-==FeCO3��+H2O+CO2�� 13.9/a
����������Һ�к��д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4������м��Fe��H2SO4������Fe2(SO4)3��Ӧ����FeSO4��TiOSO4ˮ������TiO2xH2O�����ˣ�����ΪTiO2xH2O��Fe����ҺΪFeSO4��FeSO4��Һͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ������������壬��ˮ�����յõ���������FeSO4��Һ�м�̼����泥�����̼����������������狀Ͷ�����̼��̼�����������������ܽ���������������Һ�Ͷ�����̼������������Һͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ������������塣
(1)��������������Һ�������IJ����ǹ��ˣ��ʴ�Ϊ�����ˣ�
(2)TiOSO4ˮ������TiO2xH2O�Ļ�ѧ����ʽΪ��TiOSO4+(x+1)H2O![]() TiO2xH2O��+H2SO4����м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O�������ʴ�Ϊ��TiOSO4+(x+1)H2O
TiO2xH2O��+H2SO4����м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O�������ʴ�Ϊ��TiOSO4+(x+1)H2O![]() TiO2xH2O��+H2SO4����м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O������
TiO2xH2O��+H2SO4����м��H2SO4��Ӧ��c(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O������
(3)���������ڿ��������������������������ķ���ʽΪ��4FeSO4+O2![]() 2Fe2O3+4SO3���÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ1: 4���ʴ�Ϊ��1: 4��
2Fe2O3+4SO3���÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ1: 4���ʴ�Ϊ��1: 4��
(4)����У�̼�����������ᷴӦ����������������Ӧ�����ӷ���ʽΪFeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�����ʴ�Ϊ��FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2����
(5)����ܵ����ӷ���ʽ��Fe2++2HCO3-�TFeCO3��+H2O+CO2�����ʴ�Ϊ��Fe2++2HCO3-�TFeCO3��+H2O+CO2����
(6)�������ӻᱻ�����������Ϊ���������ӣ���������ԭΪ+2�۵������ӣ����ݵ����غ㣬��5FeSO47H2O��KMnO4����������0.1000molL-1 KMnO4��Һ20.00mL�����Ծ�����FeSO47H2O����������=![]() ��100%=
��100%=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���±���25 ��ʱijЩ����ĵ���ƽ�ⳣ����
��ѧʽ | CH3COOH | HClO | H2CO3 | H2C2O4 |
Ka | Ka��1.8��10��5 | Ka��3.0��10��8 | Ka1��4.1��10��7 | Ka1��5.9��10��2 |
��1��H2C2O4�뺬�����ʵ�����KOH����Һ��Ӧ��������Һ�����ԣ�����Һ�и�����Ũ���ɴ�С��˳��Ϊ��
��2����0.1 mol��L��1 CH3COOH��Һ�еμ�NaOH��Һ��c��CH3COOH����c��CH3COO������5��9����ʱ��ҺpH����
��3����̼������Һ�еμ�������ˮ�����ӷ���ʽΪ��
��4����0.1mol��L��1CH3COOH��Һ��0.1mol��L��1NaOH��Һ�������ϣ����Ի�Ϻ���Һ����ı仯������û����Һ��pH��8����c��Na+��-c��CH3COO����=mol��L��1���ȷ����������
��5����CH3COOH��Һ�����ʯ��Ӧ�������ı����44.8L����ȫ��ͨ�뵽2L 1.5mol/L��NaOH��Һ�г�ַ�Ӧ������Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ��