��Ŀ����
12��������ѧ���ش��������⣺��1���л����������֪ʶ�����ʹ��еĻ�����
��

��ϵͳ����Ϊ��2��6-����-3-�һ����飮
��3-��-2-�����Ľṹ��ʽ��CH3CH��OH��CH��CH3��2��
�۱������׳Ƹ��ͣ�
��2�������Ŷ��л���������������ã���Ҳ���ܵ��������ŵ�Ӱ�죮
�ٱȽϷе�
 ��
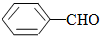
��  ���������������=������ͬ��
���������������=������ͬ���ڱȽ�ˮ���ԣ�
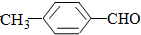 ��
��
�۱Ƚ����ԣ�
 ��CH3COOH����ʾ����ȷ��봼�����ԣ�
��CH3COOH����ʾ����ȷ��봼�����ԣ���3���ϳ��л����Ѿ��������������еõ��㷺��Ӧ�ã�
����д���ױ��ϳ�TNT�ķ�Ӧ����ʽ��
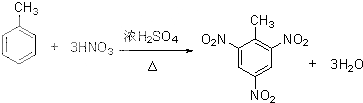 ��
������д����ȡ��ȩ��֬�ķ�Ӧ����ʽ��n
 +nHCHO
+nHCHO 
 +nH2O��
+nH2O��
���� ��1���� ���̼��Ϊ7���ֱ���2��6��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2���۱������׳Ƹ��ͣ�
���̼��Ϊ7���ֱ���2��6��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2���۱������׳Ƹ��ͣ�
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ�
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������
��3���ٰ�ŨH2SO4��ŨHNO3�ͼױ���ϼ����Ʊ�TNT���DZ�������ԭ�ӱ�����ȡ�������������ױ�������ԭ���غ���ƽ��ѧ����ʽ��
�ڷ�ȩ��֬���ɱ��Ӻͼ�ȩ�ڴ������������۶��ɣ���Ӧ�����DZ����ǻ���λ�ϵ�������ԭ�ӱȽϻ��ã����ȩȩ���ϵ���ԭ�ӽ��Ϊˮ���ӣ����ಿ������������Ϊ�߷��ӻ�����--��ȩ��֬��
��� �⣺��1���� ���̼��Ϊ7���ֱ���2��6��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��6-����-3-�һ����飬
���̼��Ϊ7���ֱ���2��6��̼�Ϻ���1��������3��̼�Ϻ���1���һ�����ȷ����Ϊ��2��6-����-3-�һ����飬
�ʴ�Ϊ��2��6-����-3-�һ����飻
��3-��-2-�����Ľṹ��ʽΪ��CH3CH��OH��CH��CH3��2��
�ʴ�Ϊ��CH3CH��OH��CH��CH3��2��
��1�����������Ӻ����������ǻ����׳Ƹ��ͣ�
�ʴ�Ϊ�����ͣ�
��2����̼ԭ����Խ�࣬�ǻ���Խ�࣬�е�Խ�ߣ��������к���3��C��3���ǻ��� �к���3��C��2���ǻ����ʱ������ķе����
�к���3��C��2���ǻ����ʱ������ķе���� ��
��
�ʴ�Ϊ��������
��ȩ��ˮ������Ȼ��ܣ�����������ˮ��������ȩ�����ܽ�ȣ�����ˮ���ԣ� ��
��
�ʴ�Ϊ����������
�۱��������Ȼ��ϵ������ӵ�����γɵĸ����Ӻͱ����Ĺ���ṹ�������ʹ����������ӱ���������Ӹ��ȶ����������Ա�����ǿ��
�ʴ�Ϊ������
��3���ٰ�ŨH2SO4��ŨHNO3�ͼױ���ϼ����Ʊ�TNT�Ļ�ѧ����ʽΪ��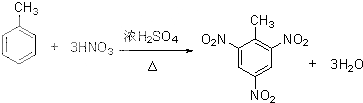 ��
��
�ʴ�Ϊ��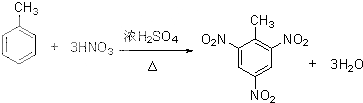 ��
��
�ڱ����ǻ���λ�ϵ�������ԭ�ӱȽϻ��ã����ȩȩ���ϵ���ԭ�ӽ��Ϊˮ���ӣ����ಿ������������Ϊ�߷��ӻ�����--��ȩ��֬����Ӧ�ķ���ʽ���Ա�ʾΪ��n +nHCHO
+nHCHO 
 +nH2O��
+nH2O��
�ʴ�Ϊ��n +nHCHO
+nHCHO 
 +nH2O��
+nH2O��
���� ������Ҫ��������л��������������ṹ��ʽ����д���л����۷е��Լ�����ǿ���ıȽϣ��л���ѧ��Ӧ����ʽ����д�ȣ��ۺ��Խ�ǿ����һ�����Ѷȣ�ע���л�������ŵ����ʣ���Ŀ�Ѷ��еȣ�

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�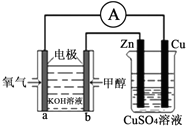 ��Դ����������������ٵ��ش���⣬�״���δ����Ҫ����Դ����֮һ�����ü״�ȼ�ϵ�������ͼ��ʾ��װ�ã�������˵���в���ȷ���ǣ�������
��Դ����������������ٵ��ش���⣬�״���δ����Ҫ����Դ����֮һ�����ü״�ȼ�ϵ�������ͼ��ʾ��װ�ã�������˵���в���ȷ���ǣ�������| A�� | ��װ����Cu��Ϊ���� | |
| B�� | ��װ�����ҳ�Ϊ��Ƴأ���������CuSO4��ҺpH���� | |
| C�� | b���ĵ缫��ӦʽΪCH3OH+8OH--6e-�TCO32-+6H2O | |
| D�� | ��ͭƬ�������仯Ϊ12.8gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ1.12L |
| A�� | ����һ��������������������ˮ������ܾ������� | |
| B�� | ��������һ����ɫ���壬������ܲ��ȶ� | |
| C�� | �����⣨H2Se������ɫ���ж�����H2S�ȶ������� | |
| D�� | ������������ˮ |
 ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯��ͼ��ʾ��������˵��������ǣ�������
ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯��ͼ��ʾ��������˵��������ǣ�������| A�� | ��Ӧ�Ļ�ѧ����ʽ��X+2Y?2Z | |
| B�� | �÷�Ӧ��0-3minʱ���ڲ���Z��ƽ����Ӧ���� 0.083mol•L-1•min-1 | |
| C�� | �÷�Ӧ�ﵽƽ��ʱ��Ӧ��X��ת���ʦ�����45% | |
| D�� | �����������䣬�����¶ȣ�������Ӧ�����������淴Ӧ���ʽ���С |
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶 | 0.160nm | 0.143nm | 0.112nm | 0.104nm | 0.066nm |
| ��Ҫ���ϼ� | +2 | +3 | +2 | +6��-2 | -2 |
| A�� | ��̬�⻯��Ļ�ԭ��ΪH2T��H2R | B�� | L2+��R2-�ĺ����������� | ||
| C�� | M��T�γɵĻ����ﲻ�������� | D�� | ������ϡ���ᷴӦ������ΪL��Q |
| A�� | �������뻹ԭ�������ʵ���֮��Ϊ2��1 | |
| B�� | ���������뻹ԭ��������ʵ���֮��Ϊ1��1 | |
| C�� | �÷�Ӧ˵��Cu�Ľ����Ժ�ǿ | |
| D�� | 2mol H2SO4���뷴Ӧʱ����4 mol���ӷ���ת�� |
| A�� | ������ϩ�����ܷ����ӳɷ�Ӧ��Ҳ�ܷ���������Ӧ | |
| B�� | ���ϡ��ͺϳ���ά�������л��߷��ӻ����� | |
| C�� | ���ۡ������ǡ�֬���͵�������һ�������¶��ܷ���ˮ�ⷴӦ | |
| D�� | ���ۡ���ά�ص�ͨʽ��Ϊ��C6H10O5��n�������Dz���Ϊͬ���칹�� |
| A�� | HBr��HCl��HF | B�� | HF��H2O��NH3 | C�� | NH3��PH3��H2S | D�� | SiH4��CH4��NH3 |