��Ŀ����
��10�֣���֪KMnO4��MnO2�����������¾��ܽ������ƣ�Na2C2O4��������
2MnO4����5C2O42����16H����2Mn2����10CO2����8H2O
MnO2��C2O42����4H����Mn2����2CO2����2H2O
ij�о�С��Ϊ�ⶨij���̿���MnO2������������ȷ��ȡ1.20g���̿���Ʒ������2.68g�����ƹ��壬�ټ���������ϡ���Ტ���ȣ����ʲ��μӷ�Ӧ������ַ�Ӧ֮����ȴ����ȥ���ʣ���������Һת�Ƶ�����ƿ�в����ݣ�����ȡ��25.00mL����Һ������ƿ�У�����0.0200mol��L��1KMnO4����Һ���еζ���������20.00mLKMnO4��Һʱǡ����ȫ��Ӧ���Իش��������⣺
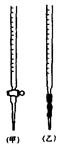
��0.0200mol��L��1KMnO4����ҺӦ���� ��ѡ��ס����ҡ����ζ����У��ζ��յ�����ж� ��
�����ܷ�������о�С��������̿���MnO2���������� ��ѡ��ܡ��������ش��ܡ�������������� �����ش𡰷���˵��ԭ�� ��
������ʵ������д������в��������л�ʹ����MnO2����������ƫС���� ��
E����ƿˮϴ֮��δ�ô���Һ��ϴ
2MnO4����5C2O42����16H����2Mn2����10CO2����8H2O
MnO2��C2O42����4H����Mn2����2CO2����2H2O
ij�о�С��Ϊ�ⶨij���̿���MnO2������������ȷ��ȡ1.20g���̿���Ʒ������2.68g�����ƹ��壬�ټ���������ϡ���Ტ���ȣ����ʲ��μӷ�Ӧ������ַ�Ӧ֮����ȴ����ȥ���ʣ���������Һת�Ƶ�����ƿ�в����ݣ�����ȡ��25.00mL����Һ������ƿ�У�����0.0200mol��L��1KMnO4����Һ���еζ���������20.00mLKMnO4��Һʱǡ����ȫ��Ӧ���Իش��������⣺

��0.0200mol��L��1KMnO4����ҺӦ���� ��ѡ��ס����ҡ����ζ����У��ζ��յ�����ж� ��
�����ܷ�������о�С��������̿���MnO2���������� ��ѡ��ܡ��������ش��ܡ�������������� �����ش𡰷���˵��ԭ�� ��
������ʵ������д������в��������л�ʹ����MnO2����������ƫС���� ��
| A����Һת��������ƿ�У�δ���ձ�������ϴ�� |
| B���ζ�ǰ���첿����һ���ݣ��ζ��յ�ʱ��ʧ |
| C������ʱ�����ӿ̶��� |
| D���ζ�ǰ���Ӷ������ζ����Ӷ��� |
�ż� ����������1��KMnO4��Һ����ƿ����Һ��������Ϻ�ɫ���Ұ�����ڲ���ɫ�����ﵽ�ζ��յ� �Ʒ� ��Ϊ��֪������ƿ�Ĺ�� ��BC
����ζ�ʵ���������������
��1�����Ը��������Һ�����ԣ��Ҿ���ǿ�����ԣ����Ӧ������ʽ�ζ����У���ʽ�ζ��ܵ��¶��Dz����ܣ���ʽ�ζ��ܵ��¶����ܣ����Լ�����ʽ�ζ��ܣ����Ǽ�ʽ�ζ��ܡ��������Ը��������Һ���Ϻ�ɫ�����Բ���Ҫָʾ�������ﵽ�յ�ʱ��Һ�����Ϻ�ɫ���ݴ˿����жϡ�
��2�����ݷ�Ӧ���̿��Լ����25.00ml��Һ�й����Ĵ����Ƶ����ʵ����������ڲ�֪������ƿ�Ĺ�������������ܵĹ����Ĵ����ƣ����Ҳ���ܼ���������̵�����������
��3��A˵�����Ƶ���Ʒ��Ũ��ƫ�ͣ�������ĵĸ�����ص�����ƫ�ͣ�������Ĵ����Ƶ����ʵ���Ҳƫ�ͣ����ԺͶ������̵ķ�Ӧ�Ĵ����ƾ�ƫ�ߣ����ⶨ���ƫ�ߡ�B��˵�����ĵĸ�����ص���ƫ�ߣ���Ͷ������̷�Ӧ�Ĵ����ƾ�ƫ�ͣ��ⶨ���ƫ�͡�C�����Ƶ���Ʒ��Ũ��ƫ�ߣ�������ĵĸ�����ص�����ƫ�ߣ�������Ĵ����Ƶ����ʵ���Ҳƫ�ߣ����ԺͶ������̵ķ�Ӧ�Ĵ����ƾ�ƫ�ͣ����ⶨ���ƫ�͡�D��˵�����ĵĸ�����ص����ƫ�ͣ�������ĵĸ�����ص�����ƫ�ͣ���������ķ�����֪�����յIJⶨ�����ƫ�ߡ���ƿ�Ͳ����ñ�Һ��ϴ������E�Dz�Ӱ��ġ���ѡBC��
��1�����Ը��������Һ�����ԣ��Ҿ���ǿ�����ԣ����Ӧ������ʽ�ζ����У���ʽ�ζ��ܵ��¶��Dz����ܣ���ʽ�ζ��ܵ��¶����ܣ����Լ�����ʽ�ζ��ܣ����Ǽ�ʽ�ζ��ܡ��������Ը��������Һ���Ϻ�ɫ�����Բ���Ҫָʾ�������ﵽ�յ�ʱ��Һ�����Ϻ�ɫ���ݴ˿����жϡ�
��2�����ݷ�Ӧ���̿��Լ����25.00ml��Һ�й����Ĵ����Ƶ����ʵ����������ڲ�֪������ƿ�Ĺ�������������ܵĹ����Ĵ����ƣ����Ҳ���ܼ���������̵�����������
��3��A˵�����Ƶ���Ʒ��Ũ��ƫ�ͣ�������ĵĸ�����ص�����ƫ�ͣ�������Ĵ����Ƶ����ʵ���Ҳƫ�ͣ����ԺͶ������̵ķ�Ӧ�Ĵ����ƾ�ƫ�ߣ����ⶨ���ƫ�ߡ�B��˵�����ĵĸ�����ص���ƫ�ߣ���Ͷ������̷�Ӧ�Ĵ����ƾ�ƫ�ͣ��ⶨ���ƫ�͡�C�����Ƶ���Ʒ��Ũ��ƫ�ߣ�������ĵĸ�����ص�����ƫ�ߣ�������Ĵ����Ƶ����ʵ���Ҳƫ�ߣ����ԺͶ������̵ķ�Ӧ�Ĵ����ƾ�ƫ�ͣ����ⶨ���ƫ�͡�D��˵�����ĵĸ�����ص����ƫ�ͣ�������ĵĸ�����ص�����ƫ�ͣ���������ķ�����֪�����յIJⶨ�����ƫ�ߡ���ƿ�Ͳ����ñ�Һ��ϴ������E�Dz�Ӱ��ġ���ѡBC��

��ϰ��ϵ�д�
�����Ŀ
 ��10��12������������Һ�������Ϻ�������Һ�ʵĸ����ӵ�Ũ���ɴ�С���е�˳����
��10��12������������Һ�������Ϻ�������Һ�ʵĸ����ӵ�Ũ���ɴ�С���е�˳����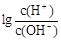 ����80��ʱ����Ba(OH)2��Һ�����AG=______________�����������������ʽ��
����80��ʱ����Ba(OH)2��Һ�����AG=______________�����������������ʽ��