��Ŀ����
��12�֣���1�������£�ˮ�����ӻ�KW=__________����һ��ˮ�У�Լ20��ˮΪ1mL��������H+��ĿΪ_________��
��2�����80��ʱ����ˮ��c(H+)=2��10��7����80��ʱŨ��Ϊ0.2 mol��L-1��Ba(OH)2��Һ��pH=______�����¶��½�����Һa L��pH=1��H2SO4��Һb L��ϣ�
�������û��ҺΪ���ԣ���a��b= ��
�������û��Һ��pH��12����a��b= ��
��Ϊ���õر�ʾ��Һ������ԣ���ѧ���������ȣ�AG���ĸ������AG=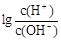 ����80��ʱ����Ba(OH)2��Һ�����AG=______________�����������������ʽ��
����80��ʱ����Ba(OH)2��Һ�����AG=______________�����������������ʽ��
��2�����80��ʱ����ˮ��c(H+)=2��10��7����80��ʱŨ��Ϊ0.2 mol��L-1��Ba(OH)2��Һ��pH=______�����¶��½�����Һa L��pH=1��H2SO4��Һb L��ϣ�
�������û��ҺΪ���ԣ���a��b= ��
�������û��Һ��pH��12����a��b= ��
��Ϊ���õر�ʾ��Һ������ԣ���ѧ���������ȣ�AG���ĸ������AG=
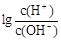 ����80��ʱ����Ba(OH)2��Һ�����AG=______________�����������������ʽ��
����80��ʱ����Ba(OH)2��Һ�����AG=______________�����������������ʽ����12�֣���1��KW=_10-14�� H+��ĿΪ 5��10-12NA ��
��2��13 �� 1:4 ����7:18 ����_2lg5-14��-12-2lg2��
��2��13 �� 1:4 ����7:18 ����_2lg5-14��-12-2lg2��
20��ˮΪ1mL����Ϊ1g,���ʵ���Ϊ0.056mol,���е�ˮ������Ϊ0.056NA, һ��ˮ��ˮ������Ϊ0.0028NA,��һ��ˮ��������H+��ĿΪ5��10-12NA. .80��ʱŨ��Ϊ0.2 mol��L-1��Ba(OH)2��Һ��C(OH-)=0.4mol��L-1 C(H+)=4��10��14��0.4=10-13
0.4a=0.1b a��b=1:4 (0.4a-0.1b)��a+b=0.04 a��b=7:18
0.4a=0.1b a��b=1:4 (0.4a-0.1b)��a+b=0.04 a��b=7:18

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
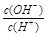 =1��10-6������������ȷ����
=1��10-6������������ȷ����