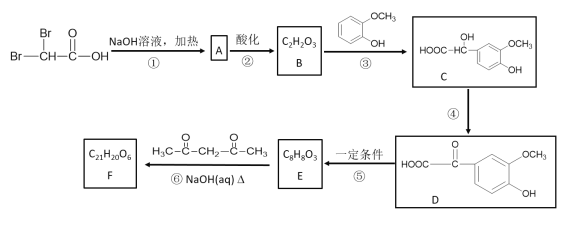
��Ŀ����
����Ŀ��Ϊʵ�� �����ܼ��š� �͡���̼���á���һ���������ν�CO2ת��Ϊ��������Դ��Ŀǰ����ҵ�ϳ���CO2������ȼ�ϼ״����ֽ�������ʵ�飺�����Ϊl L���ܱպ��������У�����l mol CO2��3mol H2��һ�������·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H����49.0 kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H����49.0 kJ/mol�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
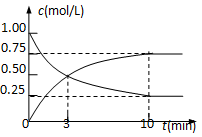
�ٸ÷�Ӧ��ƽ�ⳣ������ʽK�� ��
�ڴӷ�Ӧ��ʼ��ƽ��ʱ��CH3OH��ƽ����Ӧ����v(CH3OH)�� ��ע����λ����H2��ת���ʣ� ��
������˵���У���˵��������Ӧ�ﵽƽ��״̬����
A��ÿ����1mol CO2��ͬʱ����1mol CH3OH
B��CO2��H2��CH3OH��H2O�����ʵ����ı�Ϊ1��3��1��1
C�������������ѹǿ���ٸı�
D��������������ܶȲ��ٸı�
�����д�ʩ�У���ʹ����ƽ��״̬������Ӧ�����ƶ�����
A�������¶�
B����CH3OH(g)����ϵ�з���
C��ʹ�ø�Ч����
D�����º����ٳ���1 mol CO2��3mol H2
���𰸡���![]()
��0.075 mol��(L��min)��1��75%
��C �� BD
��������
�����������CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��ƽ�ⳣ������ʽK��
CH3OH(g)+H2O(g)��ƽ�ⳣ������ʽK��![]() ��
��
��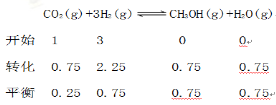
v(CH3OH)��![]() =0.075 mol��(L��min)��1��H2��ת���ʣ�
=0.075 mol��(L��min)��1��H2��ת���ʣ�![]() 75%��
75%��
������˵���У���˵��������Ӧ�ﵽƽ��״̬����
A��ÿ����1mol CO2��ͬʱ����1mol CH3OH ��![]() ��һ��ƽ�� ��
��һ��ƽ�� ��
B��CO2��H2��CH3OH��H2O�����ʵ����ı�Ϊ1��3��1��1 ����һ��ƽ�⣻
C����Ӧǰ����������ʵ����DZ����������������ѹǿ�DZ����������������ѹǿ���ٸı䣬һ��ƽ�⣻
D��![]() ���ܶ��Ǻ�����������������ܶȲ��ٸı䣬��һ��ƽ�⣻
���ܶ��Ǻ�����������������ܶȲ��ٸı䣬��һ��ƽ�⣻
�����д�ʩ�У���ʹ����ƽ��״̬������Ӧ�����ƶ�����
A���÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���
B����CH3OH(g)����ϵ�з��룬ƽ�������ƶ���
C��ʹ�ø�Ч���� ��ƽ�ⲻ�ƶ�
D�����º����ٳ���l mol CO2��3mol H2���൱�ڼ�ѹ��ƽ�������ƶ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���Թ�����Ϊ�����������������V2O5���ǽӴ�����������Ĵ������ӷϷ������л���V2O5�ȱ�����Ⱦ��������������Դ�ۺ����á��Ϸ���������Ҫ�ɷ�Ϊ��
���� | V2O5 | V2O4 | K2SO4 | SiO2 | Fe2O3 | Al2O3 |
��������/% | 2.2~2.9 | 2.8~3.1 | 22~28 | 60~65 | 1~2 | <1 |
������һ�ַϷ��������չ���·�ߣ�
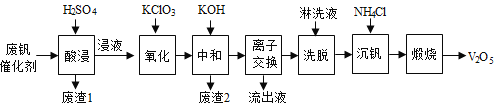
�ش��������⣺
��1���������ʱV2O5ת��ΪVO2+����Ӧ�����ӷ���ʽΪ________________________��ͬʱV2O4ת��VO2+��������1������Ҫ�ɷ���__________________��
��2��������������ʹ3 mol��VO2+��ΪVO2+������Ҫ������KClO3����Ϊ_____________mol��
��3�����к�������֮һ��ʹ����V4O124��ʽ��������Һ�С�������2���к���______________��
��4�������ӽ���������ϴ�����ɼ�ʾΪ��4ROH+ V4O124![]() R4V4O12+4OH��ROHΪǿ���������ӽ�����֬����Ϊ�����ϴ��Ч�ʣ���ϴҺӦ�ó�_____�ԣ�����������������������������
R4V4O12+4OH��ROHΪǿ���������ӽ�����֬����Ϊ�����ϴ��Ч�ʣ���ϴҺӦ�ó�_____�ԣ�����������������������������
��5�����������õ�ƫ����泥�NH4VO3��������д�����������з�����Ӧ�Ļ�ѧ����ʽ____________��