��Ŀ����
8��ʵ������Ҫ480mL 1mol•L-1 NaOH��Һ��������Һ��������ش��������⣺��1��ʵ���г���������ƽ�����룩��ҩ�ס���Ͳ���ձ������������Ҫ�����������н�ͷ�ιܡ�500mL����ƿ��
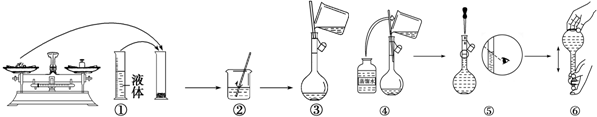
��2����ͼ��ijͬѧ��ʵ�������Ƹ�NaOH��Һ�Ĺ���ʾ��ͼ�������д�����Ǣ٢ۢݣ��������ţ���
��3����ȡNaOH����ʱ���������������ΪD����д��ĸ��
A.19.2g B.20g C.19.2g��20g D������20g
��4������Һ�����ƹ����У������»���ʵ�鲽�裬����ֻ�����һ�εIJ���������ǣ���д��������Ĵ��ţ��ڢ�
�ٳ��� ���ܽ� ��ת�� ��ϴ�� �ݶ��� ��ҡ��
��5�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ���BF
A��NaOH���峤�ڱ�¶�ڿ�����
B����ˮ�ܽ�NaOH���������ת������ƿ�ж���
C������̼���ƹ���ʱ���������ʺ�����ŷ�
D�����ݺ���Һ����ڿ��ߣ����ý�ͷ�ιܽ������ˮ����
F������ʱ���ӿ̶��ߣ�
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��2����������һ�����ʵ���Ũ����Һ����ȷ�����������������ȷʹ�÷������
��3������n=cV��m=nM�����500mL1.0mol/L ��NaOH��Һ�к��������������Ƶ�������Ȼ������������Ƶij���Ҫ�ŵ�С�ձ��������������
��4������ʵ������ķ��������жϣ�
��5������ʵ�������c=$\frac{n}{V}$��Ӱ�������������
��� �⣺��1�����������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ�
�����ṩ��������֪������������500ml����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����ͷ�ιܡ�500mL����ƿ��
��2������ͲΪ��ȡ���������������ܽ����ʣ��ʢٴ���
���ò�����������ٹ�����ܽ⣬�ʢ���ȷ��
�۲�������������ʱ���������¶�Ӧ��������ƿ�̶����·����ʢ۴���
�ܼ�ˮ���̶��ߵ��·���������ȷ���ʢ���ȷ��
�ݶ���ʱ���۾�Ӧƽ�ӿ̶��ߣ��ʢݴ���
�Ӹ�ҡ�ȣ�ʹ��Һ��Ͼ��ȣ�������ȷ���ʢ���ȷ��
�ʴ�Ϊ���٢ۢݣ�
��3��������450mL������ƿ����ѡ��500mL������ƿ�����Ƴ�500mL��1.0mol/L����Һ��500mL 1.0mol/L������������Һ�к������ʵ�����Ϊ��m=1.0mol/L��0.5L��40g/mol=20g����Ҫ�������������Ƶ�����Ϊ20.0g���������������ƹ���ʱ��Ҫ�ŵ�С�ձ����������ѡ�õ��������������20.0g��
�ʴ�Ϊ��D��
��4���ٳ���ʱ�ȳƿ��ձ��������ٳ��ձ���ҩƷ���������ʢٴ���
�ڹ������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У�ֻ��1�Σ��ʢ���ȷ��
��ת��ʱ���˽���Һת�Ƶ�����ƿ�л�Ҫ��ϴ��Һת�Ƶ�����ƿ�У��ʢڴ���
��ϴ��ʱҪϴ���ձ���������2-3�Σ��ʢܴ���
�ݶ���ʱ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ��ֻ��1�Σ�����ȷ��
��ҡ��Ҫ����Һ��ҡ��һ�Σ��ڶ��ݺ���ҡ��һ�Σ��ʢݴ���
��ѡ���ڢݣ�
��5��A��NaOH���峤�ڱ�¶�ڿ����У����²����������Ʊ��ʣ����Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ�Ũ��ƫ�ͣ���A����
B����ˮ�ܽ�NaOH���������ת������ƿ�ж��ݣ��ȵ���Һ���ƫ����ȴ�������С�������Ƶ���Һ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ���B��ȷ��
C������̼���ƹ���ʱ���������ʺ�����ŷ������������������Ƶ�����ƫС�����Ƶ���ҺŨ��ƫ�ͣ���C����
D�����ݺ���Һ����ڿ��ߣ����ý�ͷ�ιܽ������ˮ���������Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���D����
F������ʱ���ӿ̶��ߣ����¼��������ˮ���ƫС�����Ƶ���Һ���ƫС�������Ƶ���ҺŨ��ƫ�ߣ���F��ȷ��
�ʴ�Ϊ��BF��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�ѶȲ���ע������һ�����ʵ���Ũ�ȵ���Һ������ʵ��IJ�����������5��Ϊ�ѵ㡢�״��㣬��Ҫ��ȷ���ƹ������������ķ�����

| A�� | ȩ���Ľṹ��ʽ-COH | B�� | ������ӵı���ģ��Ϊ��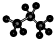 | ||
| C�� | ���Ȼ�̼���ӵĵ���ʽΪ 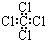 | D�� | 2-�һ�-1��3-����ϩ�ļ���ʽ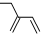 |
| A�� | ������ͬ��Ŀ��ԭ�� | B�� | ��ռ22.4 L | ||
| C�� | ������ͬ��Ŀ�ķ��� | D�� | ������ͬĦ������ |
| A�� | 0.5 mol | B�� | 0.6 mol | C�� | 0.7 mol | D�� | 0.8 mol |
| A�� | ��ͭ��ͭп�Ͻ�������ͭ�����ͭ�� | |
| B�� | ������������о�������Ǵ������������� | |
| C�� | ��п��ϡ���ᷴӦʱ������������ͭ��Һ�����ʼӿ� | |
| D�� | ���ʽ��ƾ��ú����䰵 |
| A�� | �����������������Ƿ���ʣ�����$\stackrel{�ܽ�}{��}$��Һ$\stackrel{���ᱵ��Һ}{��}$��ɫ����$\stackrel{ϡ����}{��}$�������ܽ� | |
| B�� | ��ȥ���������л��е�������Һ$\stackrel{����̼������Һ��}{��}$��Һ�ֲ�$\stackrel{��Һ}{��}$���ϲ�Һ�� | |
| C�� | ֤������������H2O2�����Ա�I2ǿ��NaI��Һ$\stackrel{30%H_{2}O_{2}��Һϡ���ἰ����}{��}$��Һ����ɫ | |
| D�� | ������A�ijɷ���FeBr2��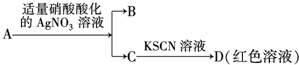 |
 ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺