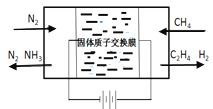
��Ŀ����
����Ŀ����֪����1molH2(g)�е�H��H����Ҫ����436kJ����������1molI2(g)�е�I��I����Ҫ����151kJ����������1molHI(g)�е�H��I���ܷų�299kJ����������˵����ȷ���ǣ� ��
A.1molH2(g)��1molI2(g)��������Ϊ587kJ
B.H2(g)+I2(s)![]() 2HI(g) ��H=-11kJ��mol-1
2HI(g) ��H=-11kJ��mol-1
C.HI(g)![]()
![]() H2(g)+
H2(g)+![]() I2(g) ��H=+5.5kJmol-1
I2(g) ��H=+5.5kJmol-1
D.I2(g)��H2�����ȶ�
���𰸡�C
��������
A. ������Ϣ�ɼ��㷴Ӧ����ܼ��ܣ�
B. ������Ϣ�ɼ��������͵�������Ӧ�ķ�Ӧ�ȣ�
C. ������H=��Ӧ����ܼ���-��������ܼ��ܽ��м��㣻
D. ����Խ������Խ�ȶ���
A.������Ϣ���ܼ���1molH2(g)��1molI2(g)����������A�����
B.���״̬Ϊ����ʱ��H2(g)+I2(g)![]() 2HI(g) ��H=-11kJ��mol-1��B�����
2HI(g) ��H=-11kJ��mol-1��B�����
C.�⻯��ֽ����ȣ�HI(g)![]()
![]() H2(g)+
H2(g)+![]() I2(g)��C����ȷ��
I2(g)��C����ȷ��
D.��Խ��Խ�ȶ���H2��I2(g)�����ȶ���D�����
��ѡC��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����֪����1mol������к���2molSi��Si����
��Si(s)��O2(g)===SiO2(g) ��H���䷴Ӧ�����������仯��ͼ��ʾ��
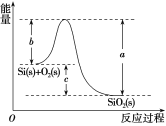
��
��ѧ�� | Si��O | O=O | Si��Si |
�Ͽ�1 mol���ۼ���������/kJ | 460 | 500 | 176 |
����˵������ȷ���ǣ� ��
A.������������ǽ���ѧ��ת��Ϊ����B.���������ȶ���С�ڹ���ȶ���
C.��H����988kJ��mol��1D.��H��(a��c)kJ��mol��1