��Ŀ����
����Ŀ�������仯�������ճ�������������;�㷺������FeSO4�Ʊ���ԭ���۵Ĺ�ҵ�������£�
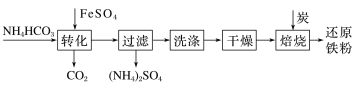
ʵ�����п���FeSO4(�����ۺ�ϡ���ᷴӦ�Ƶ�)��NH4HCO3��Һ������װ��ģ�����������е���ת�������ڡ�
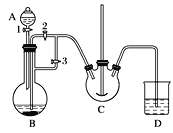
(1)װ��A������������________��װ��B��ʢ�ŵ�ҩƷ��________��
(2)ʵ������У��������ɵ�FeSO4��Һ��NH4HCO3��Һ��ϣ�����������____________________��FeSO4��ҺҪ���������Ƶ�ԭ����_____________________��
(3)������̵���ҪĿ������ȥ����FeCO3�е�����ˮ���ù����л�������FeCO3�ڿ����б�����ΪFeOOH���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________________��ȡ������FeCO3��Ʒ24.98 g����̿��Ϻ��գ����յõ���ԭ����12.32 g��������Ʒ��FeCO3����������________%(������ȡ����)��
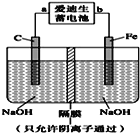
(4)��������(Na2FeO4)��һ�����;�ˮ��.����ͼװ�ÿ�����ȡ�����������ƣ�д�����õ�ⷨ��ȡ��������ʱ�������ĵ缫��Ӧʽ___________________
(5)25 ��ʱ��Ksp[Fe(OH)3]��4.0��10��38 �����¶��£���ʵ����������2.5mol/L 100mL��������Һ��Ϊʹ���ƹ����в����ֻ��ǣ���������Ҫ����10mol/L������______mL(���Լ�����������)��
���𰸡���Һ©�� ���� ��D�������������رջ���3������2 ���������ױ����� 4FeCO3��O2��2H2O===4FeOOH��4CO2 93 ![]() 0.25
0.25
��������
A����Һ©������ʢ�ŵ���ϡ���ᣬB��ʢ�ŵ������ۣ�C��ʢ�ŵ���NH4HCO3��Һ����ʼ��Һ©���Ͽڲ�����������1������3���رջ���2����Ӧ���е�װ���еĿ����ž����رջ���3������2��B�����ɵ�����������Һ��ѹ��C����̼�������Һ��Ӧ�����ɳ������������ˣ�ϴ�ӣ�������յû�ԭ���ۡ�
(1)A��Bװ����������ȡ���������ģ�����ϡ���ᷴӦ��������������������
(2)ʵ������У���D�������������رջ���3������2���ɽ�B�����ɵ�FeSO4��Һ��NH4HCO3��Һ��ϣ� ���������ξ���ǿ��ԭ�����ױ������е�������������������������ã�
(3)�������Ļ��ϼ۷����˱仯��ȷ����Ӧ��ΪFeCO3��O2��H2O������ΪFeOOH��CO2д������ʽ4FeCO3��O2��2H2O===4FeOOH��4CO2�����ù�ϵʽ������FeCO3����������
(4)����ͼ����Կ���������ʧ���������������ӽ�����ɸ���������ӣ�д���缫��Ӧʽ��
(5)��������������Ksp���м��㡣
(1)A��Bװ����������ȡ���������ģ�����ϡ���ᷴӦ��������������������
��ȷ�𰸣���Һ©�� ���ۡ�
(2)ʵ������У���D�������������رջ���3������2���ɽ�B�����ɵ�FeSO4��Һ��NH4HCO3��Һ��ϣ����������ξ���ǿ��ԭ�����ױ������е�������������������������ã�
��ȷ�𰸣���D�������������رջ���3������2�����������ױ�������
(3)�������Ļ��ϼ۷����˱仯��ȷ����Ӧ��ΪFeCO3��O2��H2O������ΪFeOOH��CO2д������ʽ4FeCO3��O2��2H2O===4FeOOH��4CO2�����ù�ϵʽ������FeCO3������������
FeCO3 �� Fe FeOOH �� Fe
116g 56g 89g 56g
m��FeCO3��g m��FeOOH��g
������֪�ã�m��FeCO3��+ m��FeOOH��=24.98 g��![]() +
+![]() =12.32g;
=12.32g;
������ʽ�����ɵó�m��FeCO3��=23.2g m��FeOOH��=1.78g
��Ʒ��FeCO3����������:![]() ��100%��93%;
��100%��93%;
��ȷ��: 4FeCO3��O2��2H2O===4FeOOH��4CO2 93��
(4)����ͼ����Կ���������ʧ���������������ӽ�����ɸ���������ӣ�д���缫��Ӧʽ![]() ��
��
��ȷ�𰸣�![]()
(5)����2.5mol/L 100mL��������Һ����������������Ksp���м��㣺Ksp[Fe(OH)3]��C��Fe3+����![]() =5��
=5��![]() = 4.0��10��38��C��OH-��=2��10��13mol/L������C��H+��=Kw/ C��OH-��=5��10��2mol/L������C1V1=C2V2����֪5��10��2mol/L��100��10��3L=20��V1��10��3L��V1=0.25mL��
= 4.0��10��38��C��OH-��=2��10��13mol/L������C��H+��=Kw/ C��OH-��=5��10��2mol/L������C1V1=C2V2����֪5��10��2mol/L��100��10��3L=20��V1��10��3L��V1=0.25mL��
��ȷ�𰸣�0.25��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��һ���¶��£���2L�ܱ������з������з�Ӧ��4NO2(g)+O2(g)![]() 2N2O5(g)����֪�÷�Ӧ��ƽ�ⳣ����K300����K350��������ϵ��n(NO2)(��λ��mol)��ʱ��仯���±���
2N2O5(g)����֪�÷�Ӧ��ƽ�ⳣ����K300����K350��������ϵ��n(NO2)(��λ��mol)��ʱ��仯���±���
ʱ��(s) | 0 | 500 | 1000 | 1500 |
t1�� | 20 | 13.96 | 10.08 | 10.08 |
t2�� | 20 | a | b | c |
����˵��һ����ȷ����
A. ����ӦΪ���ȷ�Ӧ
B. ���t2�棼t1�棬��ôa��b=c����a=10+0.5b
C. ���t2�棼t1�棬��ôt2��ﵽƽ���ʱ�����1000s��1500s֮��
D. ���t2�棾t1�棬��ôb��10.08
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ�����
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | M������6������ |
X | �����������Ǵ�����������2�� |
Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
Z | Ԫ����������ǣ�7�� |
W | �䵥�ʼ��ܸ��ᷴӦ�����ܸ�ǿ�Ӧ��������H2 |
��1��Ԫ��X��һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����_____��
��2��Ԫ��Y����Ԫ���γ�һ������YH4����д�������ĵ���ʽ_____����μ���ij��Һ�к�������_____��
��3��Ԫ��Z�����ڱ��е�λ��_____��Ԫ��Z��Ԫ��T��ȣ��ǽ����Խ�ǿ����_____����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����_____������ţ���
a.������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b.Z���⻯���T���⻯���ȶ�
c.һ�������£�Z��T�ĵ��ʶ���������������Һ��Ӧ







