��Ŀ����
2��ij�о�С��Ϊ��̽��S02��ʵ�����Ʒ����йػ�ѧ���ʣ���������µ�ʵ�飮ʵ��I��
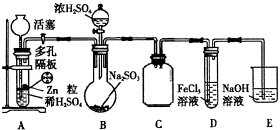
��1��ʵ���������������
�ٶ�������������ϣ����������ǿ��Կ��Ʒ�Ӧ��ʱ����Ҳ������ʱֹͣ��
�ڸ�ʵ����H2ʱ����Ũ��������480mL 3mol/L��ϡ���ᣬ����������IJ���������250mL�ձ���l 00mL��Ͳ������������ͷ�ιܵȣ�����500mL������ƿ��
�۸�ʵ�������H2����������ǸϾ�װ���еĿ�����
��2��ʵ��ʱ�ȴ�������װ��A���Լ���Ӧһ��ʱ��رջ���������װ��B�е�������Ũ���ᷴӦƬ�̺ס�����ѧ���ֱ�ȡװ��D��������Һ����ѧ�������м������Ը�KMnO4��Һ���۲쵽����KMn O4��Һ�Ϻ�ɫ��ȥ����ѧ�������м��������ữ��BaCl2��Һ���۲쵽�а�ɫ�������ɣ����ݸ���ʵ������ס���ѧ���ó�SO2��Fe3+������
�����жϼס���ѧ���Ľ����Ƿ��Ͻ�B����дѡ���
A����ѧ���Ͻ� B����ѧ���Ͻ� C���ס���ѧ�������Ͻ�
��д��S02��Fe3+���������ӷ�Ӧ����ʽSO2+2Fe3++2H2O=2Fe2++SO42-+4H+��
ʵ��II��
��3�����о�С��Ϊ�ⶨSO2������ΪSO3��ת���ʣ������������ʵ�飺��֪SO3�۵�Ϊ16.8�棬�Һ��Կ�����CO2��Ӱ�죩��
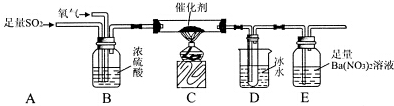
�ٵ�ֹͣͨ��SO2��Ϩ��ƾ��ƺ���Ҫ����ͨ����������Ŀ���ǣ�ʹ�д���װ���еĶ��������������������գ�
��ʵ���������װ��D���ӵ�����Ϊm g��װ��E�в�����ɫ����������Ϊn g����������¶��������ת������$\frac{m}{80}$�£�$\frac{m}{80}$+$\frac{n}{233}$����100%���ú���ĸ�Ĵ���ʽ��ʾ�����û���
���� ��1���ٶ�������������ϣ��ܹ������շ������Ĺ��ܣ�
������480mL 3mol/L��ϡ���ᣬ��Ҫ500mL������ƿ��
��A����ȡ������Ŀ���ǸϾ�װ���еĿ����������ռ������������
��2������Һ�еĶ�������Ҳ�ܹ�ʹ���Ը��������Һ��ɫ��
�������ӽ�������������Ϊ�������������ԭΪ�������ӣ�
��3���ٵ�ֹͣͨ��SO2��Ϩ��ƾ��ƺ�װ��BCDE�л���û�����յ����壻
��װ��D�����ӵ����������������������װ��E�а�ɫ���������������ᱵ���������ݴ˷�����
��� �⣺��1���ٶ�������������ϣ��ܹ������շ������Ĺ��ܣ��ʴ�Ϊ�����Կ��Ʒ�Ӧ��ʱ����Ҳ������ʱֹͣ��
������480mL 3mol/L��ϡ���ᣬ��Ҫ500mL������ƿ��250mL�ձ���l00mL��Ͳ������������ͷ�ιܣ��ʴ�Ϊ��500mL������ƿ��
��A����ȡ������Ŀ���ǸϾ�װ���еĿ����������ռ�����������ʴ�Ϊ���Ͼ�װ���еĿ�����
��2����װ��D�����ж�������ʱ����Һ�еĶ�������Ҳ�ܹ�ʹ���Ը��������Һ��ɫ�����Լ�ѧ�����Ͻ�����ѧ��������������ӣ��Ͻ����ʴ�Ϊ��B��
�������ӽ�������������Ϊ�������������ԭΪ�������ӣ����ӷ���ʽΪSO2+2Fe3++2H2O=2Fe2++SO42-+4H+���ʴ�Ϊ��SO2+2Fe3++2H2O=2Fe2++SO42-+4H+��
��3���ٵ�ֹͣͨ��SO2��Ϩ��ƾ��ƺ�װ��BCDE�л���û�����յ����壬����ͨ��������ʹ�д���װ���еĶ��������������������գ�
�ʴ�Ϊ��ʹ�д���װ���еĶ��������������������գ�
��װ��D�����ӵ���������������������������������ʵ���Ϊ$\frac{m}{80}$mol��װ��E�а�ɫ���������������ᱵ��������n��SO2��=n��BaSO4��=$\frac{n}{233}$mol�����Զ��������ת����Ϊ$\frac{m}{80}$�£�$\frac{m}{80}$+$\frac{n}{233}$����100%���ʴ�Ϊ��$\frac{m}{80}$�£�$\frac{m}{80}$+$\frac{n}{233}$����100%��
���� ���⿼�������շ�����ԭ������Һ����������ʹ�á�������ԭ��Ӧ����ʽ��д�Լ���������ʵ�飬��Ŀ�ѶȽϴ�

| A�� | ԭ�Ӱ뾶��X��Y��Z | B�� | ԭ��������X��Y��Z | ||
| C�� | ��̬�⻯���ȶ��ԣ�X��Y��Z | D�� | �ǽ����ԣ�X��Y��Z |
| ��� | �Լ�B | ʪ�����ֽA | ���� |
| A | ��ˮ | ������ֽ���� | ����������� |
| B | Ũ��ˮ����ʯ�� | ��ɫʯ����ֽ��� | ����Ϊ�������� |
| C | Na2SO3������ | Ʒ����ֽ��ɫ | SO2����Ư���� |
| D | Cu��Ũ���� | ����KI��ֽ���� | NO2Ϊ�������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 �շ���͡��һ�ֵ���Ѫ֬��ҩ���ṹ��ʽ��ͼ��ʾ��δ��ʾ����ռ乹�ͣ������й����շ���͡������������ȷ���ǣ�������
�շ���͡��һ�ֵ���Ѫ֬��ҩ���ṹ��ʽ��ͼ��ʾ��δ��ʾ����ռ乹�ͣ������й����շ���͡������������ȷ���ǣ�������| A�� | ����FeCl3 ��Һ������ɫ��Ӧ | |
| B�� | ��ʹ����KMnO4 ��Һ��ɫ | |
| C�� | �ܷ����ӳɡ�ȡ����������ȥ��Ӧ | |
| D�� | 1 mol ������������1 mol NaOH ��Ӧ |
| A�� | KNO3��Һ�л�������K2SO4����������BaCl2��Һ | |
| B�� | ������̼�����л���������������ͨ�����Ը��������Һ���ռ����� | |
| C�� | ��ȥFeCl2��Һ�л��е�FeCl3������������ۣ����� | |
| D�� | ��ȥCu���л��е�CuO��������ϡ������ˡ�ϴ�� |
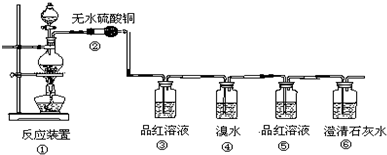
 SO2�����ʵ��о����Ʊ�����������Ҫ����;���ȿ�����Ϊԭ���ֿ���Ϊ�ܼ�����ش��������⣺
SO2�����ʵ��о����Ʊ�����������Ҫ����;���ȿ�����Ϊԭ���ֿ���Ϊ�ܼ�����ش��������⣺