��Ŀ����
A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ����������������֪����Aԭ�Ӱ뾶��̣���Bԭ�������������Ǵ�����������2������Cԭ�������������ȴ�����������4������Dԭ�ӵĴ�����������������������8������E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ����F��Cͬ���壮�ش��������⣺
��1��������Ԫ���У��γ��������������� ����Ԫ�����ƣ���
��2��д��CԪ�����е��⻯��ĵ���ʽ ��
��3���õ���ʽ��ʾD2F���γɹ��� ��
��4��A2C�ķе��A2F�ķе�ߵ���Ҫԭ�� ��
��5��д��E���ռ���Һ��Ӧ�����ӷ���ʽ ��
��1��������Ԫ���У��γ���������������
��2��д��CԪ�����е��⻯��ĵ���ʽ
��3���õ���ʽ��ʾD2F���γɹ���
��4��A2C�ķе��A2F�ķе�ߵ���Ҫԭ��
��5��д��E���ռ���Һ��Ӧ�����ӷ���ʽ
������A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������Bԭ�������������Ǵ�����������2������B��CԪ�أ���Aԭ�Ӱ뾶��̣�С��BԪ�أ���A��HԪ�أ� ��Cԭ�������������ȴ�����������4������C��OԪ�أ���Dԭ�ӵĴ�����������������������8������D��ԭ����������C�Ķ�����Ԫ�أ�����D��NaԪ�أ���E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ������E��AlԪ�أ���F��Cͬ���壬��F��ԭ����������E�Ķ�����Ԫ�أ�����F��SԪ�أ�
����⣺A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������Bԭ�������������Ǵ�����������2������B��CԪ�أ���Aԭ�Ӱ뾶��̣�С��BԪ�أ���A��HԪ�أ� ��Cԭ�������������ȴ�����������4������C��OԪ�أ���Dԭ�ӵĴ�����������������������8������D��ԭ����������C�Ķ�����Ԫ�أ�����D��NaԪ�أ���E���ʼ��ܺ����ᷴӦ���ܺ��ռӦ������E��AlԪ�أ���F��Cͬ���壬��F��ԭ����������E�Ķ�����Ԫ�أ�����F��SԪ�أ�
��1��������Ԫ���У��γ���������������̼Ԫ�أ��ʴ�Ϊ��̼��
��2��C��OԪ�أ����γɵ��⻯����ˮ��˫��ˮ�������ʽ�ֱ�Ϊ�� ��
��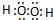 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��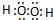 ��
��
��3�������γɹ����У���ԭ�Ӱ��������Ӹ���ԭ�ӣ�ʹ�����Ӻ������Ӷ��ﵽ�ȶ��ṹ���������γɹ���Ϊ��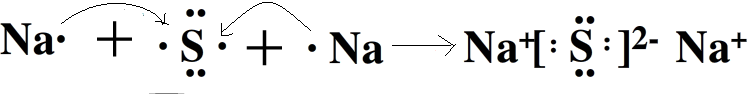 ��
��
�ʴ�Ϊ��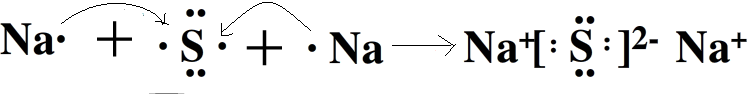 ��
��
��4��H2O�ķе��H2S�ķе�ߣ�ԭ����ˮ���Ӽ����������������в����ǻ����ʴ�Ϊ��ˮ���Ӽ���������
��5����������������Һ��Ӧ����ƫ�����ƺ������������ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
��1��������Ԫ���У��γ���������������̼Ԫ�أ��ʴ�Ϊ��̼��
��2��C��OԪ�أ����γɵ��⻯����ˮ��˫��ˮ�������ʽ�ֱ�Ϊ��
 ��
��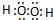 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��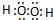 ��
����3�������γɹ����У���ԭ�Ӱ��������Ӹ���ԭ�ӣ�ʹ�����Ӻ������Ӷ��ﵽ�ȶ��ṹ���������γɹ���Ϊ��
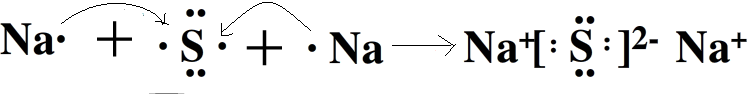 ��
���ʴ�Ϊ��
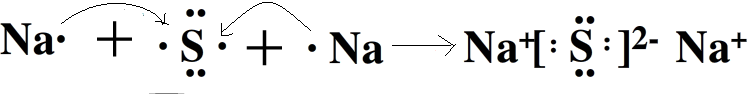 ��
����4��H2O�ķе��H2S�ķе�ߣ�ԭ����ˮ���Ӽ����������������в����ǻ����ʴ�Ϊ��ˮ���Ӽ���������
��5����������������Һ��Ӧ����ƫ�����ƺ������������ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
���������⿼�����ӷ���ʽ������ʽ����д��֪ʶ�㣬��ȷ�ƶ�Ԫ���ǽⱾ��ؼ�������ԭ�ӽṹ�������⣬�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�